Introduction
Urination or micturition removes metabolic products and toxic wastes filtered from the kidneys and is a vital human bodily function. The micturition reflex requires a complex network of signals between the nervous system and the urinary tract. Urine storage and bladder emptying are highly dependent on these pathways.
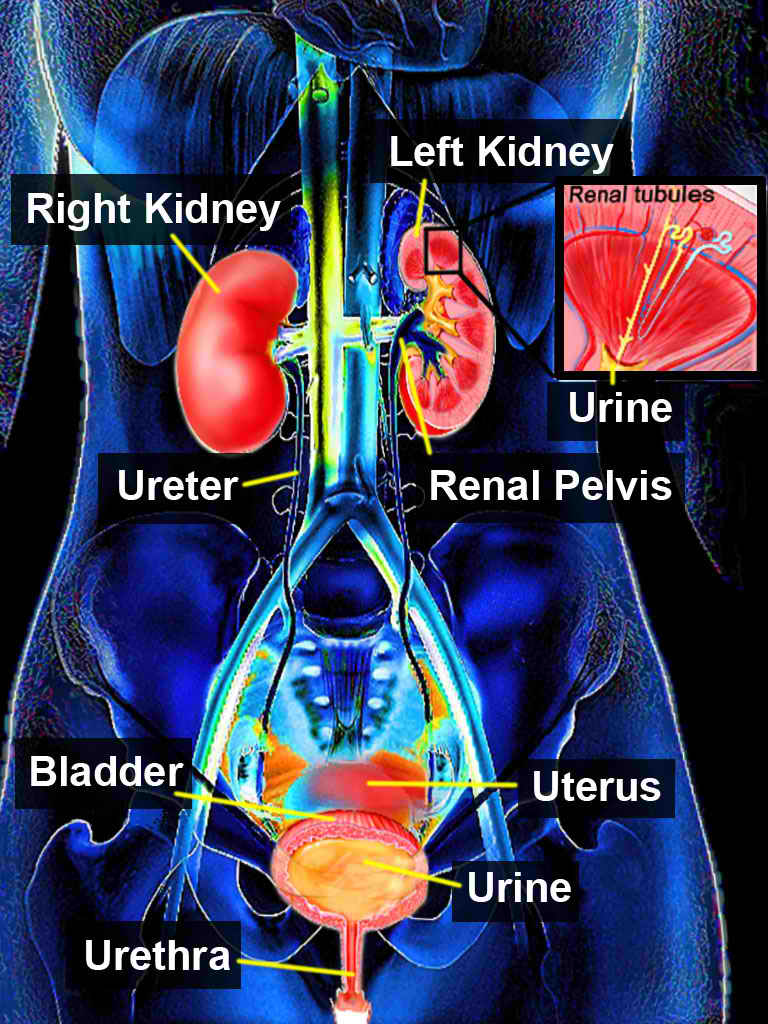
Neurological function and anatomic abnormalities can involve many disease states and disorders, such as incontinence, bladder outlet obstruction, urinary retention, vesicoureteral reflux, incomplete emptying, detrusor overactivity, or infections (see Image. Urinary System).
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Understanding the process of normal micturition is critical to managing disorders of abnormal urination. Issues of concern related to the physiology of urination encompass a range of conditions and complications that can significantly impact an individual's urinary system and overall well-being. These issues include urinary incontinence, where voluntary control over urination is compromised, affecting individuals across various age groups. Urinary retention, characterized by the inability to empty the bladder fully, can lead to discomfort and potential complications like urinary tract infections. Frequent and urgent urination, often associated with conditions like overactive bladder, can disrupt daily life and activities. Hesitancy, or difficulty initiating urination, may indicate underlying problems such as an enlarged prostate in men.
Painful urination can be a symptom of various conditions like urinary tract infections or interstitial cystitis, causing discomfort during urination. Neurological disorders, like spinal cord injuries or multiple sclerosis, can affect the nerves that control urination. Structural abnormalities in the urinary tract, congenital or acquired, can contribute to urinary issues. Hormonal changes, particularly in women during menopause, can also impact urinary function.
Additionally, certain medications can have urinary problems as an adverse effect, and inadequate fluid intake leading to concentrated urine can exacerbate these concerns. Addressing these issues requires a comprehensive assessment, accurate diagnosis, and the development of individualized treatment plans tailored to the underlying cause of the problem, aiming to improve urinary function and overall quality of life.
Cellular Level
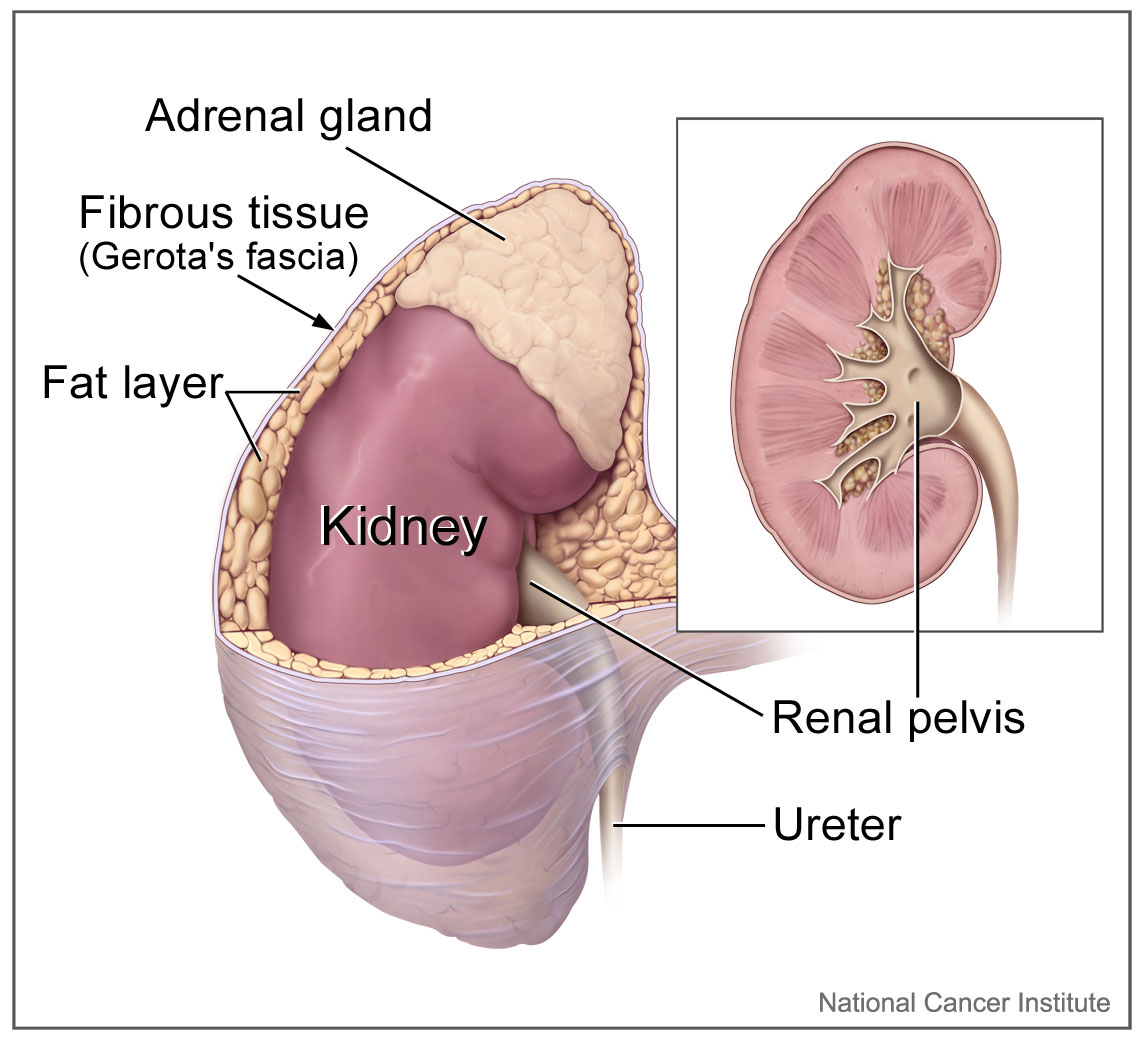
The upper urinary-collecting system consists of the kidneys, of which the renal papillae are the first gross structure of the upper urinary tract (see Image. Kidney Gross Anatomy). These papillae are individually cupped by the minor calyx, which then goes to the infundibulum and combines to form the major calyces. The major calyces then coalesce to form the renal pelvis. Urine flows from the renal pelvis into the bilateral ureters, consisting of fibromuscular tubes that terminate in the bladder (see Image. Transverse Section of Ureter). The ureters are narrowest at the ureteropelvic junction, ureterovesical junction, and where they cross the common iliac vessels, which is clinically significant.[1]
The lower urinary tract system consists of the bladder and urethra (see Image. Median Sagittal Section of Male Pelvis Anatomy). The urethra connects with the bladder neck and is the final destination for urine to flow through before exiting the body.[2][3]
The histological anatomy of the urinary tract comprises a specialized epithelium called urothelium (transitional epithelium), characterized by cell surface glycoproteins known as uroplakins.[4] These uroplakins cover most of each urothelial cell and are preferentially directed toward the luminal interior.[4]
Different parts of the urinary tract have distinctive epithelium (see Image. Urinary Bladder, Vertical Section of Bladder Wall). Some areas, such as the ureters, contain fewer uroplakins and cytoplasmic fusiform vesicles than the bladder urothelium. The bladder is mostly composed of smooth muscle and collagen with elastic and nerve fibers.[5][6][7] The bladder also contains a large number of "umbrella cells," which are big, multinucleated luminal cells that can form large urothelial plaques. They are in direct contact with urine and are found in the most superficial mucosal layer of the renal pelvis, ureters, and especially the urinary bladder.
Umbrella cells are primarily responsible for the initial barrier to mucosal penetration by urine, ions, and solutes. They must maintain this defense during all phases of stretching as the bladder fills and empties.[8] This is accomplished by changing their physical shape and stretching as the bladder fills.[8] Adjacent umbrella cells form tight junctions and membrane interdigitations, further contributing to their barrier function.[9]
The bladder urothelium has a unique lipid and protein composition that forms an apical membrane, high-resistance tight junctions with strong intercellular interdigitations, and can regulate the movement of water, ions, and solutes through the mucosal surface.[10] The urothelium can also produce mucus and release chemical mediators and neurotransmitters that communicate with and affect underlying nerve and muscle tissues.[10][11]
Recent research suggests that urothelial cells can also initiate bladder contractions via local signaling or sensory nerve stimulation.[12] This finding expands our understanding of the complex regulatory processes involved in bladder function and has potential implications for developing treatments for various urinary disorders.
Development
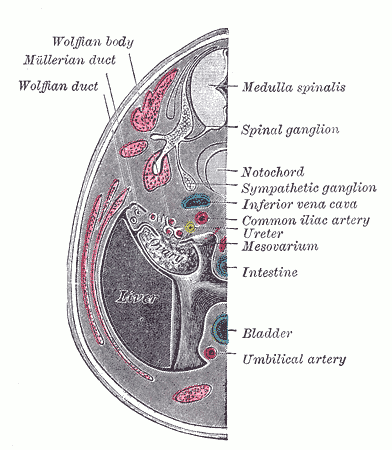
The development of the urinary tract system starts at week 4, with the pronephros as a set of rudimentary and nonfunctional kidneys.[13] These disappear and pave the way for the mesonephros, which function from week 5 through week 10 as the permanent kidneys form (see Image. Urogenital Apparatus, Human Embryo Aged 8.5 to 9 Weeks). The mesonephric structures make a pathway for urine production.[13] The metanephros, a critical stage in kidney development, emerges during fetal development around week 5. While it begins to form during this early stage, it becomes fully functional by approximately week 10. Subsequently, the metanephros continues to mature and develop, ultimately evolving into fully functional adult kidneys.[13]
The metanephros comprises 2 essential components: the metanephric mesenchyme, where nephrogenesis, or the formation of nephrons (the functional units of the kidney), occurs, and the ureteric bud. The ureteric bud is responsible for giving rise to the collecting system, which includes structures such as the collecting tubules, major and minor calyces, renal pelves, and ureters.[13][14]
Function
Urination or micturition primarily functions in the excretion of metabolic products and toxic wastes. The urinary tract also serves as a storage vessel for the liquid waste filtered from the kidneys. Urine stored in the urinary bladder is released and expelled through the urethra by a complex network of coordinated neurological functions.
The bladder muscle comprises 3 layers that are significantly interwoven and intermingled. The inner and outer layers are mainly longitudinal, while the intermediate layer comprises circular fibers.[15]
The bladder can accommodate increasing amounts of urine without significantly changing its low intravesical pressure until it fills or a detrusor contraction occurs. A normal bladder maintains an intravesical pressure of 20 cm H2O or less during filling until it reaches fullness or contracts.[16] This ability of the bladder to manage increasing amounts of urine without increasing pressure is called compliance. A loss of bladder compliance results in urinary frequency and incontinence.[17]
Compliance can be calculated mathematically as Compliance=ΔV(mL)/ΔP(cm H2O), with values of 12.5 to 40 mL/cm H2O generally considered normal.[17][18][19] Lower values (<12.5 mL/cm H2O) indicate a loss of compliance as intravesical pressure would increase significantly with bladder filling by relatively small volumes.[17]
Loss of bladder compliance can result from bladder neurogenicity (eg, from multiple sclerosis, cauda equina syndrome, myelodysplasia), overactivity, detrusor instability, irritability, radiation effect, chronic denervation, long-term bladder outlet obstruction, infectious and noninfectious cystitis, possibly from tuberculosis or interstitial cystitis.[20]
Patients with bladder overactivity show a rise in detrusor pressure even when bladder filling is stopped, but those with a loss of compliance drop their intravesical pressure when bladder filling ceases.[17] Medical treatment of loss of compliance is based on maintaining the sustained detrusor pressure at <40 cm H2O, which not only improves storage but also helps protect the kidneys and upper tracts by protecting the ureterovesical junction (UVJ) and avoiding vesicoureteral reflux.[17] The management of loss of compliance may involve anticholinergics/antimuscarinics, β-adrenergic medications, sacral neuromodulation, tibial nerve stimulation, onabotulinum toxin A detrusor injections, intermittent self-catheterization, and augmentation cystoplasty.[17]
The first sensation of bladder filling is typically noted at 150 mL to 250 mL in normal adults. Fullness is generally appreciated at 350 mL to 400 mL but can be easily suppressed by voluntary activity of the pudendal nerve. The maximum normal capacity is about 500 mL. A detrusor pressure of 30 to 40 cm H2O is sufficient for bladder emptying in men, less in women.
Mechanism
The brain, spinal cord, and peripheral ganglia all affect the micturition reflex. Afferent pathways from the bladder to the brain include the dorsal system and the spinothalamic tract. Efferent pathways from the brain to the bladder exist for micturition and storage, which regulate the bladder and an outlet, ie, the bladder neck, urethra, and urethral sphincter.
Bladder Storage
Bladder storage is maintained by the pontine storage center, which activates and maintains spinal sympathetic tone to the bladder and somatic stimulation of the urethral sphincter.[21]
Sympathetic fibers originating from the T11-L2 spinal segments travel in the hypogastric nerve and link to the base of the bladder and urethra through the β3 adrenergic and α1 adrenergic receptors, respectively. Their primary function is facilitating bladder storage. When sympathetic postganglionic neurons release noradrenaline, the β3 receptors are stimulated to relax the bladder smooth muscle, while the α1 receptors activate and contract the urethral smooth muscle to help store urine.[21]
Somatic motor nerves from the S2-S4 motor neurons travel in the pudendal nerves and link to the striated muscles of the external urethral sphincter through the nicotinic cholinergic receptor. The release of acetylcholine from the somatic axons stimulates the nicotinic receptors of the external sphincter and causes them to contract, facilitating bladder storage.[21]
Guarding Reflex
The "guarding reflex" also helps maintain continence during situations that suddenly increase intravesical pressure, such as coughing, sneezing, straining, Valsalva maneuver, and laughing. Such a sudden increase in intravesical pressure stimulates the bladder wall's stretch-sensitive mechanoreceptors, which then send afferent signals to the sacral spinal cord and Onuf's nucleus (the sacral origin of the pudendal nerve, located in the anterior horn of S2.) Efferent signals from Onuf's nucleus are then transmitted via the pudendal nerve and stimulate contraction of the external sphincter muscle, preserving continence.[22][23] The process is normally automatic, quick, effective, and involuntary.[21][22][23]
Bladder Emptying or Voiding
Voiding starts with increased afferent activity from the detrusor muscle. This activity stimulates the pontine micturition center, which blocks the guarding reflex described above. The pontine micturition center, also called Barrington's nucleus, is located in the brainstem, where it coordinates neurological activity from the periaqueductal gray matter and elsewhere that results in voiding.[21][24][25]
The pelvic floor and sphincteric muscles relax by voluntary action of the somatic nerves. Sympathetic tone is reduced, and parasympathetic stimulation begins. Parasympathetic preganglionic fibers originate from the S2-S4 spinal segments and travel in the pelvic nerves. They link to the bladder wall through M3 muscarinic receptors. When the parasympathetic postganglionic axons release acetylcholine, the M3 receptors of the bladder smooth muscle (detrusor) are stimulated, and the bladder contracts. These combined pathways conjunctively lead to normal urination.[21][26][27][28]
Spinal cord lesions above the pontine voiding centers result in coordinated voiding reflex functions, which are lost if the lesions are below this level. In such situations, detrusor-sphincter-dyssynergia may result.[29] See StatPearls' companion reference on Bladder Sphincter Dyssynergia.[29]
Bladder Storage and Emptying Reflexes Summary
Storage: Low-level vesical afferent stimulation is produced during urinary storage as the bladder fills and distends. This action stimulates the hypogastric nerve (T11-L2), which provides continuous sympathetic tone to the bladder, inducing detrusor relaxation and promoting urinary storage. Somatic innervation stimulation through the pudendal nerve (S2-S4) is also stimulated, resulting in urethral sphincter closure, further facilitating continence and bladder storage. The pontine storage center is activated, helping to maintain urethral sphincter tone and activity.[21]
Voiding: Intense vesical afferent stimulation of the pelvic nerves activates the spinobulbospinal reflex pathways, the periaqueductal gray matter, and the pontine micturition center. The guarding reflex is suppressed, as well as sympathetic (relaxing) tone to the bladder and pudendal somatic urethral closure. Parasympathetic stimulation utilizing the pelvic nerves (S2-S4) initiates bladder contractility while inhibiting somatic activity. The detrusor contracts and the urethral sphincter opens, allowing micturition and urinary flow with bladder emptying. The pontine storage center and the periaqueductal gray matter provide higher neurological control to coordinate these processes. Higher brain centers initiate voluntary and conscious voiding.[30]
Clinical Significance
Pathologies affecting the urinary tract system can result from both intrinsic and extrinsic factors. These conditions encompass a wide range of disorders, including neurological disorders, outlet obstruction, and infections. Neurological disorders can disrupt the normal coordination of bladder function, leading to issues such as urinary incontinence or retention. Outlet obstruction, often caused by conditions like an enlarged prostate in men or pelvic organ prolapse in women, can impede urine flow. Infections, which can be bacterial or viral, can affect various parts of the urinary tract, leading to infections or pyelonephritis. Understanding the underlying causes of urinary tract pathologies is crucial for accurate diagnosis and effective treatment.
Incontinence
Incontinence is common in older patients, patients with diabetes, overactive bladder, and postmenopausal women.[31] See StatPearls' companion reference on Urinary Incontinence.[31]
- Urge incontinence characteristically presents as the sudden need to pass urine, with the underlying cause most likely idiopathic, although excessive caffeine intake and infections should be excluded first.[32]
- Stress incontinence is voiding after increased intrabdominal pressure, such as from coughing or sneezing, most likely from dysfunctional urethral closure.[33] See StatPearls' companion reference on Stress Incontinence.[33]
- Mixed urinary incontinence is a condition combining both stress and urge incontinence.[34][35] See StatPearls' reference on Mixed Urinary Incontinence.[35]
- Overflow incontinence is an increase in post-void residual urine due to incomplete bladder emptying or urinary retention.[36] See StatPearls' references on Female Urinary Retention and Male Urinary Retention.[36][37]
The narrowing of the ureter at certain anatomical locations may become obstructed by ureteral calculi.[1][38] The most common places are at the ureteropelvic junction, ureterovesical junction, and when they cross the common iliac vessels.[1][2][38] Common signs and symptoms of ureterolithiasis or nephrolithiasis include hematuria, severe intermittent colicky flank pain radiating to the groin, nausea, and vomiting.[38]
Congenital Abnormalities
The complex nature of genitourinary embryology can lead to various congenital abnormalities, such as unilateral renal agenesis, due to the abnormal interaction of the ureteric bud and metanephric mesenchyme.[13] See Statpearls' companion reference on Genitourinary Embryology.[13]
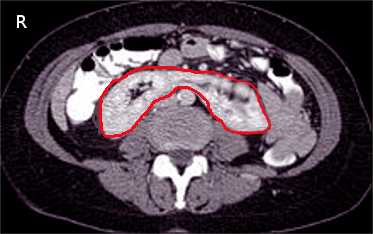
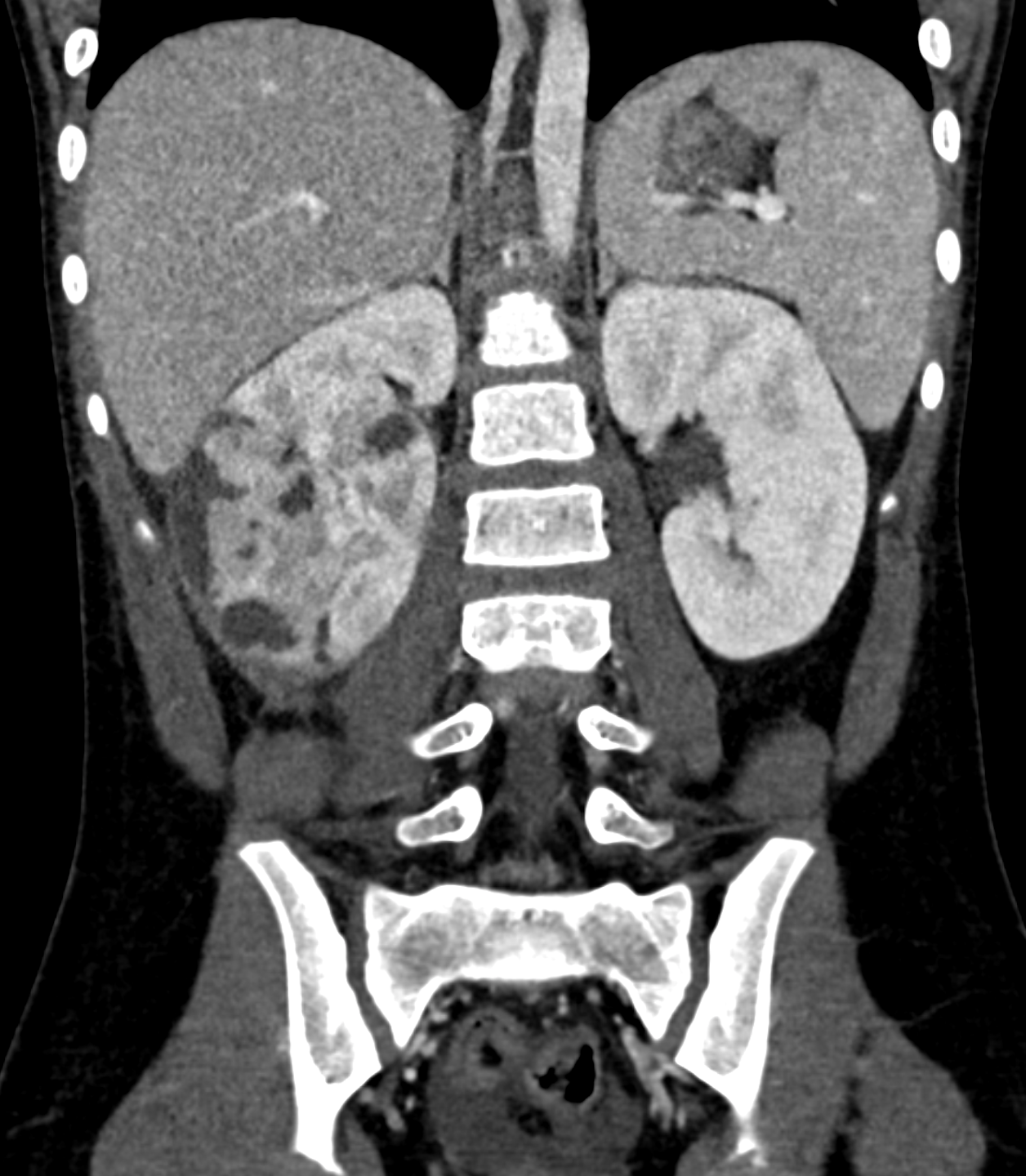
Horseshoe kidneys occur when the inferior poles of both kidneys fuse, most commonly during ascent (see Image. CT Scan, Horseshoe Kidney).[39][40] This condition can often be found in Turner syndrome (see Image. Girl With Turner Syndrome).[39][41] See StatPearls' companion reference on Horseshoe Kidneys and Turner Syndrome.[40][41]
Infections
Due to its anatomy and physiological function, the genitourinary tract is prone to infections, urinary tract infections.[42]
- Urethritis is more common in females because of the urethra's shorter length and proximity to the anus than in males.[43]
- Cystitis is bladder inflammation and can present with suprapubic pain, urinary urgency, and frequency.[44]
- Prostatitis is inflammation of the prostate, usually characterized by male pelvic discomfort and other symptoms.[45]
- Pyelonephritis is inflammation of the renal parenchyma and pelvis (see Image. Pyelonephritis With Renal Abscesses).[46] The condition can present with upper urinary tract infection symptoms such as fever, flank pain, costovertebral angle tenderness, and lower urinary tract symptomatology such as dysuria, urinary urgency, and frequency.[46][47] See StatPearls' references on Acute Pyelonephritis and Complicated Urinary Tract Infections.[46][48]
Urination holds vital clinical significance as it is essential for eliminating waste products, maintaining fluid balance, and assessing kidney function. Changes in urine composition can signal various medical conditions, including infections and metabolic disorders. Urine also plays a critical role in medication excretion, and monitoring urination patterns aids in diagnosing and managing bladder-related conditions. In essence, urination serves as a valuable diagnostic and therapeutic tool, contributing significantly to clinical assessments and treatment decisions.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
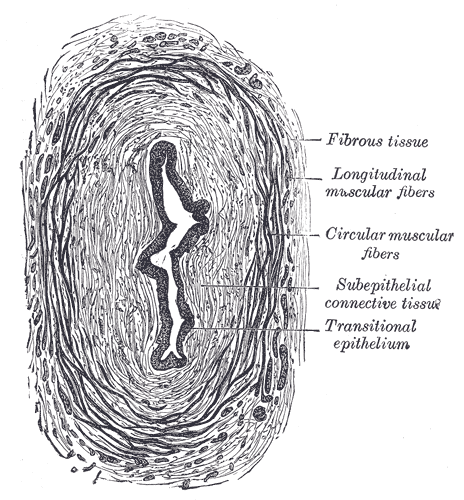
Transverse Section of the Ureter. The transverse section of the ureter's anatomy includes fibrous tissue, longitudinal muscular fibers, circular muscular fibers, subepithelial connective tissue, and transitional epithelium.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
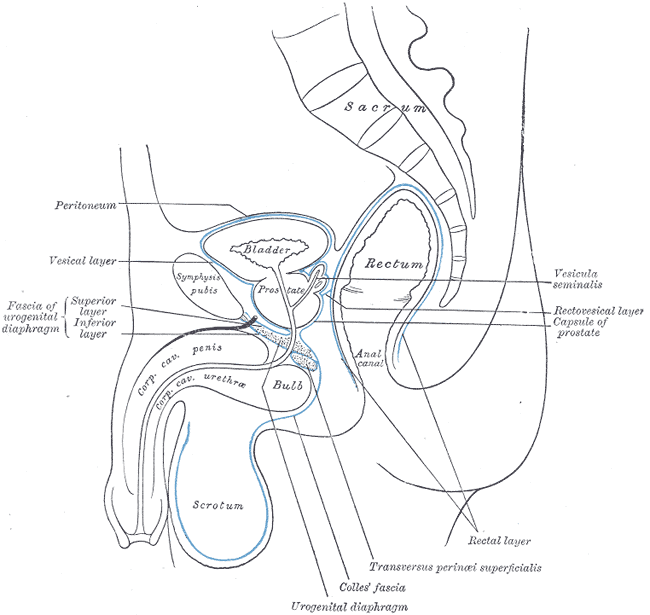
Median Sagittal Section of Male Pelvis Anatomy. Anatomy includes pelvis, sacrum, peritoneum, vesical layer, fascia of urogenital diaphragm, superior and inferior layer, bladder, prostate, symphysis pubis, rectum, anal canal, corpus cavernosum penis and urethra, bulb, scrotum, urogenital diaphragm, Colles fascia, transversus perinei superficialis, rectal layer, vesicula seminalis, rectovesical layer, and capsule of prostate.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
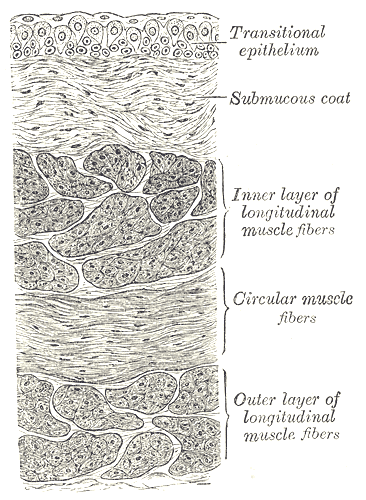
Urinary Bladder, Vertical Section of Bladder Wall. Anatomy includes transitional epithelium, submucous coat, inner layer of longitudinal muscle fibers, circular muscle fibers, and outer layer of longitudinal muscle fibers.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Lescay HA, Jiang J, Leslie SW, Tuma F. Anatomy, Abdomen and Pelvis Ureter. StatPearls. 2024 Jan:(): [PubMed PMID: 30422575]
Hickling DR, Sun TT, Wu XR. Anatomy and Physiology of the Urinary Tract: Relation to Host Defense and Microbial Infection. Microbiology spectrum. 2015 Aug:3(4):. doi: 10.1128/microbiolspec.UTI-0016-2012. Epub [PubMed PMID: 26350322]
Elbadawi A. Functional anatomy of the organs of micturition. The Urologic clinics of North America. 1996 May:23(2):177-210 [PubMed PMID: 8659020]
Matuszewski MA, Tupikowski K, Dołowy Ł, Szymańska B, Dembowski J, Zdrojowy R. Uroplakins and their potential applications in urology. Central European journal of urology. 2016:69(3):252-257 [PubMed PMID: 27729990]
Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng FM, Wu XR, Shapiro E, Sun TT. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. The Journal of cell biology. 2005 Dec 5:171(5):835-44 [PubMed PMID: 16330712]
Level 3 (low-level) evidenceRomih R, Korosec P, de Mello W Jr, Jezernik K. Differentiation of epithelial cells in the urinary tract. Cell and tissue research. 2005 May:320(2):259-68 [PubMed PMID: 15778856]
Level 3 (low-level) evidenceBolla SR, Odeluga N, Amraei R, Jetti R. Histology, Bladder. StatPearls. 2023 Jan:(): [PubMed PMID: 31082007]
Carattino MD, Prakasam HS, Ruiz WG, Clayton DR, McGuire M, Gallo LI, Apodaca G. Bladder filling and voiding affect umbrella cell tight junction organization and function. American journal of physiology. Renal physiology. 2013 Oct 15:305(8):F1158-68. doi: 10.1152/ajprenal.00282.2013. Epub 2013 Jul 24 [PubMed PMID: 23884145]
Level 3 (low-level) evidenceKreplak L, Wang H, Aebi U, Kong XP. Atomic force microscopy of Mammalian urothelial surface. Journal of molecular biology. 2007 Nov 23:374(2):365-73 [PubMed PMID: 17936789]
Level 3 (low-level) evidenceKhandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. American journal of physiology. Renal physiology. 2009 Dec:297(6):F1477-501. doi: 10.1152/ajprenal.00327.2009. Epub 2009 Jul 8 [PubMed PMID: 19587142]
Level 3 (low-level) evidenceBirder LA, Andersson KE, Kanai AJ, Hanna-Mitchell AT, Fry CH. Urothelial mucosal signaling and the overactive bladder-ICI-RS 2013. Neurourology and urodynamics. 2014 Jun:33(5):597-601. doi: 10.1002/nau.22604. Epub 2014 May 16 [PubMed PMID: 24838393]
Level 3 (low-level) evidenceRobilotto GL, Yang OJ, Alom F, Johnson RD, Mickle AD. Optogenetic urothelial cell stimulation induces bladder contractions and pelvic nerve afferent firing. American journal of physiology. Renal physiology. 2023 Aug 1:325(2):F150-F163. doi: 10.1152/ajprenal.00035.2023. Epub 2023 Jun 15 [PubMed PMID: 37318991]
Libretti S, Aeddula NR. Embryology, Genitourinary. StatPearls. 2024 Jan:(): [PubMed PMID: 32644735]
de Bakker BS, van den Hoff MJB, Vize PD, Oostra RJ. The Pronephros; a Fresh Perspective. Integrative and comparative biology. 2019 Jul 1:59(1):29-47. doi: 10.1093/icb/icz001. Epub [PubMed PMID: 30649320]
Level 2 (mid-level) evidenceShermadou ES, Rahman S, Leslie SW. Anatomy, Abdomen and Pelvis: Bladder. StatPearls. 2023 Jan:(): [PubMed PMID: 30285360]
Yao M, Simoes A. Urodynamic Testing and Interpretation. StatPearls. 2023 Jan:(): [PubMed PMID: 32965981]
Arunachalam D, Heit M. Low Bladder Compliance in Women: A Clinical Overview. Female pelvic medicine & reconstructive surgery. 2020 Apr:26(4):263-269. doi: 10.1097/SPV.0000000000000666. Epub [PubMed PMID: 30520742]
Level 3 (low-level) evidenceToppercer A, Tetreault JP. Compliance of the bladder: an attempt to establish normal values. Urology. 1979 Aug:14(2):204-5 [PubMed PMID: 473478]
Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. The Journal of urology. 2000 Apr:163(4):1228-33 [PubMed PMID: 10737503]
Level 2 (mid-level) evidenceChartier-Kastler E, Comperat E, Ruffion A. [Disorders of bladder compliance and neurogenic bladder]. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2007 May:17(3):442-7 [PubMed PMID: 17622074]
Chai TC. Continence and micturition: physiological mechanisms under behavioral control. American journal of physiology. Renal physiology. 2015 Jul 1:309(1):F33-4. doi: 10.1152/ajprenal.00193.2015. Epub 2015 May 13 [PubMed PMID: 25972511]
Chancellor MB, Yoshimura N. Neurophysiology of stress urinary incontinence. Reviews in urology. 2004:6 Suppl 3(Suppl 3):S19-28 [PubMed PMID: 16985861]
Park JM, Bloom DA, McGuire EJ. The guarding reflex revisited. British journal of urology. 1997 Dec:80(6):940-5 [PubMed PMID: 9439415]
Rahman M, Siddik AB. Neuroanatomy, Pontine Micturition Center. StatPearls. 2024 Jan:(): [PubMed PMID: 32491351]
Mokhtar M, Singh P. Neuroanatomy, Periaqueductal Gray. StatPearls. 2023 Jan:(): [PubMed PMID: 32119278]
de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Comprehensive Physiology. 2015 Jan:5(1):327-96. doi: 10.1002/cphy.c130056. Epub [PubMed PMID: 25589273]
Level 3 (low-level) evidenceSugaya K, Nishijima S, Miyazato M, Ogawa Y. Central nervous control of micturition and urine storage. Journal of smooth muscle research = Nihon Heikatsukin Gakkai kikanshi. 2005 Jun:41(3):117-32 [PubMed PMID: 16006745]
Level 3 (low-level) evidenceZachoval R, Záleský M, Lukes M, Mares J, Urban M, Palascak P. [Lower urinary tract function and its disorders]. Ceskoslovenska fysiologie. 2000 Aug:49(3):134-44 [PubMed PMID: 11039243]
Feloney MP, Leslie SW. Bladder Sphincter Dyssynergia. StatPearls. 2024 Jan:(): [PubMed PMID: 32965837]
Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature reviews. Neuroscience. 2008 Jun:9(6):453-66. doi: 10.1038/nrn2401. Epub [PubMed PMID: 18490916]
Level 3 (low-level) evidenceTran LN, Puckett Y. Urinary Incontinence. StatPearls. 2023 Jan:(): [PubMed PMID: 32644521]
Nandy S, Ranganathan S. Urge Incontinence. StatPearls. 2023 Jan:(): [PubMed PMID: 33085319]
Lugo T, Riggs J. Stress Incontinence. StatPearls. 2023 Jan:(): [PubMed PMID: 30969591]
Lukacz ES, Santiago-Lastra Y, Albo ME, Brubaker L. Urinary Incontinence in Women: A Review. JAMA. 2017 Oct 24:318(16):1592-1604. doi: 10.1001/jama.2017.12137. Epub [PubMed PMID: 29067433]
Harris S, Riggs J. Mixed Urinary Incontinence. StatPearls. 2023 Jan:(): [PubMed PMID: 30480967]
Leslie SW, Rawla P, Dougherty JM. Female Urinary Retention. StatPearls. 2024 Jan:(): [PubMed PMID: 30860732]
Dougherty JM, Aeddula NR. Male Urinary Retention. StatPearls. 2024 Jan:(): [PubMed PMID: 30860734]
Patti L, Leslie SW. Acute Renal Colic. StatPearls. 2024 Jan:(): [PubMed PMID: 28613743]
Taghavi K, Kirkpatrick J, Mirjalili SA. The horseshoe kidney: Surgical anatomy and embryology. Journal of pediatric urology. 2016 Oct:12(5):275-280. doi: 10.1016/j.jpurol.2016.04.033. Epub 2016 May 31 [PubMed PMID: 27324557]
Kirkpatrick JJ, Leslie SW. Horseshoe Kidney. StatPearls. 2024 Jan:(): [PubMed PMID: 28613757]
Shankar Kikkeri N, Nagalli S. Turner Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 32119508]
Li R, Leslie SW. Cystitis. StatPearls. 2024 Jan:(): [PubMed PMID: 29494042]
Territo H, Ashurst JV. Nongonococcal Urethritis. StatPearls. 2023 Jan:(): [PubMed PMID: 30571032]
Lala V, Leslie SW, Minter DA. Acute Cystitis. StatPearls. 2024 Jan:(): [PubMed PMID: 29083726]
Khattak AS, Raison N, Hawazie A, Khan A, Brunckhorst O, Ahmed K. Contemporary Management of Chronic Prostatitis. Cureus. 2021 Dec:13(12):e20243. doi: 10.7759/cureus.20243. Epub 2021 Dec 7 [PubMed PMID: 35004057]
Belyayeva M, Jeong JM. Acute Pyelonephritis. StatPearls. 2023 Jan:(): [PubMed PMID: 30137822]
Ramakrishnan K, Scheid DC. Diagnosis and management of acute pyelonephritis in adults. American family physician. 2005 Mar 1:71(5):933-42 [PubMed PMID: 15768623]
Sabih A, Leslie SW. Complicated Urinary Tract Infections. StatPearls. 2024 Jan:(): [PubMed PMID: 28613784]