Anatomy, Bony Pelvis and Lower Limb, Knee Anterior Cruciate Ligament
Anatomy, Bony Pelvis and Lower Limb, Knee Anterior Cruciate Ligament
Introduction
The anterior cruciate ligament (ACL) is one of the two cruciate ligaments which stabilizes the knee joint by preventing excessive forward movements of the tibia or limiting rotational knee movements. It is one of the most commonly injured structures in sports medicine, and yet it, unfortunately, does not heal when damaged.[1] This article presents the anatomy and function of the ACL to help readers better understand the injury mechanism and reduce the risk of ACL damage.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Structure
The ACL is a band of specialized connective tissue located in the knee joint that connects the femur and the tibia. They are predominantly made up of collagen fibers, which make up 70% of their dry weight. Mostly type I collagen (90%) and a small amount of Type III collagen(10%).[1] Fibroblasts make up for the cellular components embedded in a matrix of elastin (<5%) and proteoglycans[2]. On the femoral side, the ACL attaches to the posterior aspect of the medial surface of the lateral femoral condyle, which is semi-circular in shape, measuring 20 mm by 10 mm. From the femoral attachment, the ACL descends and attaches to the site anterior and lateral to the medial intercondylar tubercle.[3][4] The ligament is the narrowest near the femoral attachment and fans out and widens as it proceeds to the tibial attachment, approximately 15mm anterior to the PCL, a broader and oval-shaped footprint measuring 10mm by 30mm.[5]
The ACL is composed of two bundles: the anteromedial (AMB) and the posterolateral (PLB). The AMB fascicles arise from the most posterior and proximal site of the femoral attachment and attach to the anteromedial portion of the tibial attachment. The PLB fascicles arise from the distal portion of the femoral attachment and attach to the posterolateral portion of the tibial attachment. The ACL is mostly composed of the PLB.[6] With knee motion, the length and orientation of the AMB and PLB bundles change. With knee flexion, the AMB lengthens and tightens while the PLB shortens and becomes lax. With knee extension, the PLB lengthens and tightens while the AMB remains tight but less so than the PLB.[7] The mean length of the ACL is 33 mm, and the mean mid-substance width is 11 mm.[8]
Function
The ACL is a crucial element in stabilizing the knee joint. It primarily stabilizes the anterior tibial translation and plays a small role in resisting internal rotation and prevents any excessive movements.
Anterior Tibial Translation
The ACL functions as the primary restriction to anterior tibial translation with respect to the femur. When the knee is extended, the anterior tibial translation is low (maximum 2 mm) and supports the knee while standing. With knee flexion, the anterior tibial translation can increase up to 3 mm while walking and up to approximately 6 mm under anterior load.[9] Patients with chronic ACL-deficient knees (grade 3 sprain) experience the anterior tibial movement relative to the femur that is about four times greater than those with healthy knees.[10] A study by Zantop et al. showed that the damaged ACL increased the anterior tibial translation by up to 10 to 15 mm at 30 degrees of flexion under the anterior load of 134 N.[11] In cadaveric knees with no active muscular forces, researchers observed that the highest increase in the anterior tibial translation was between 15 to 40 degrees of knee flexion. The ischio-crural muscle group, which includes the biceps femoris, the semitendinosus, and the semimembranosus, induces knee flexion by connecting the ischial tuberosity with the pes anserinus tibia and fibular head. At 90 degrees of knee flexion, the forces of the muscle group actively stabilize against the anterior tibial translation.[12] In cadaveric knees, the PLB plays an important role in stabilizing the anterior tibial translation at near-to-extension angles, whereas the AMB is more involved in stabilizing higher flexion angles.[13][14]
Internal rotation
The ACL also functions as the secondary restraint to internal rotation, especially when the joint is close to full extension. In a study by Fleming et al., an internal torque of the tibia between 0-10 Nm resulted in the ACL strain in vivo.[15] A study by Beynnon et al. demonstrated that an internal torque between 2 to 6 Nm resulted in the AMB strain when the knee was flexed at 90 degrees in vivo.[16] The ACL may also serve as the secondary restraint to external rotation and varus-valgus angulation.[1]
Embryology
The knee starts to develop from a mesenchymal concentration at four weeks gestational age. A knee joint is identifiable starting at six weeks, and the ACL formation as a mesenchymal condensation in the fetal blastoma is observable between 6 to 7 gestational weeks. Both the posterior cruciate ligament (PCL) and ACL appear as well-oriented structures by nine weeks of gestation. The ACL initially begins as a ventral ligament but migrates posteriorly in the knee joint as the intercondylar space develops. The PCL does not move from its initial position. Both cruciate ligaments are intracapsular but extra-synovial.[17][1]
Blood Supply and Lymphatics
The middle genicular arterial (MCA) branches supply nutrients to the ACL.[1] The MCA branches arise from the anterolateral aspect of the popliteal artery located in the popliteal fossa and directly penetrate the posterior capsule.[7][18] The MCA generally originates at the level of the proximal femoral condyles and can be identified between the superior genicular artery and the sural artery.[19][1]
The lateral and medial inferior geniculate arterial branches vascularize the distal portions of the two cruciate ligaments.[20] The ACL is covered by a synovial sheath where the small branches of the inferior and middle geniculate arteries combine to form a periligamentous plexus. The periligamentous plexus horizontally penetrates the ACL to anastomose with a vertically oriented intraligamentous vascular network.[21]
Nerves
The posterior articular branches of the tibial nerve are the major neural structure that innervates the ACL.[1] These nerves penetrate the posterior capsule and travel anteriorly with the synovial and periligamentous arterial branches toward the infrapatellar fat pad. Most of these nerve fibers have a vasomotor function, but some smaller myelinated and unmyelinated nerve fibers that lie among the ligament fascicles run independently of the vessels.[22]
A few neural mechanoreceptors are located within the ACL, and they are primarily Ruffini receptors, which function as stretch receptors, and free nerve-endings, which function as nociceptors. The free nerve-endings also function as local effectors and secrete neuropeptides with vasoactive function.[23] These receptors also play a significant role in proprioception as well.
Muscles
Females are reported to suffer two- to seven-fold ACL injuries than their male counterparts of the same age. Excessive loads on ACLs per unit body weight are expected in females due to the lesser stiffness of the knee muscles.[24] In an extensive and detailed study by Hewett et al., both male and female young athletes were assessed for a decade using coupled biomechanical-epidemiological approaches.[24] The study found that the female players had four neuromuscular imbalances, namely, ligament dominance, quadriceps dominance, leg dominance, and trunk dominance.
Physiologic Variants
A biomechanical study of the cadaveric ACL showed the maximum load at failure, stiffness, and modulus of elasticity was lower in the ACLs from female cadavers than that of male cadavers.[24] That suggests that there could be a variation in the composition of the ACL between males and females.
Females are more likely to rupture their ACLs, between 2 to 7 fold higher than males of the same age.[25] It appears to be multifactorial. Studies have shown that women have higher risks of injuries in the first half of the menstrual cycle during the pre-ovulatory phase due to increased laxity of ligaments. The narrow intercondylar notch and the tibial slope does also play a significant role in increasing the risk of ACL ruptures.
Surgical Considerations
Though there are small anatomic variations in the size and location of the femoral and tibial attachment, the surgical aim in the reconstruction of ACL is to provide adequate stability to the knee while allowing it to function well, allowing the patient to return to their normal activities.
A good understanding of the anatomy of attachments, the direction of the two bundles of ACL, and its status during flexion and extension of the knee is vital in achieving correct tunnel placement during surgery for securing the graft and obtain good tensioning of the graft.
The graft used to reconstruct the ACL can be either from the patient's tissues, a cadaveric tissue, or a synthetic material. The commonest autografts used are hamstring tendon, bone-patellar tendon-bone graft, and quadriceps tendon.
The chemical treatment and the irradiated autografts carry slightly higher risks of failure, infection, and lower outcome scores compared to autografts.
Chalmers et al. reviewed 29 studies with at least a 10-year follow-up comparing surgical and conservative management of ACL injuries. Surgical reconstruction was associated with fewer meniscal injuries and subsequent surgeries than nonoperative treatment. Some also argue that there were no differences in outcome scores (IKDC scores, Tegner scores, and Lysholm scores), and radiographic arthritis between the two groups.[25]
Clinical Significance
The three modes of ACL injuries are direct contact, indirect contact, and non-contact. Approximately 30% of ACL injuries are contact injuries, and 5% of those are from indirect contact. Approximately 70% of the injuries are non-contact, which can be caused by wrong movements.[26] Non-contact injuries, which are the most common, occur due to forces generated within a person's body.
ACL tear is frequently associated with a sudden directional or speed change while the foot remains firmly planted, rapid deceleration, jumping, pivoting, and direct impact to the anterior aspect of the tibia.
An ACL injury can further classify as a grade I, II, or III sprain.
- Grade I: The ligamental fibers are stretched, with a tear that is less than one-third of the ligament. Mild tenderness and swelling are present. The knee joint feels stable with a knee laxity < 5 mm.
- Grade II: A partial tear (between one-third to two-thirds of the ligamental fibers) is present. Mild tenderness and swelling with some loss of function are present. The joint may feel unstable with increased anterior translation (a knee laxity of 5 to 10 mm). The patient feels pain, and the pain may become exacerbated with Lachman's and anterior drawer stress tests.
- Grade III: The fibers have completely torn. Tenderness and limited pain (relative to the seriousness of the injury) are features. The degree of swelling may be variable. The knee feels unstable, with rotational instability (positive pivot shift test). A knee laxity is greater than 10 mm. Haemarthrosis (bleeding into the knee joints) is observable within 1 to 2 hours.[24]
An acute ACL rupture commonly occurs among sports players, especially those aged 14 to 19 years.[27] The incidence of ACL injury is higher among female athletes due to the following reasons[27]:
- Smaller ACL and narrower intercondylar notch: Females who are non-athletes and aged 41 to 65 are predisposed to ACL injuries if they have narrow intercondylar notches.[28]
- Wider pelvis and greater Q angle: A wider pelvis increases the angle of the femur toward the central patella. The greater the Q angle, the greater pressure is applied to the medial aspect of the knee, which can lead to an ACL tear.
- Lax ligaments: Female ligaments with more elastic muscle fibers tend to be laxer than male ligaments. Excessive joint movements with increased flexibility may contribute to the higher incidence of ACL injury among females.[29]
- Greater quadriceps to hamstring strength ratio: Females tend to have poor hamstring strength compared to men. The imbalance of strength between the hamstring and quadriceps muscles may increase the risk of ACL injury.[30][31]
Presentation:
- a sensation of painful "pop" during the impact
- immediate swelling of knee/haemarthrosis
- painful and restricted range of movements in the acute phase
- often associated with medial meniscal tears
- 'giving way' or instability symptoms, during later stages, when they attempt to return to their original activities
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
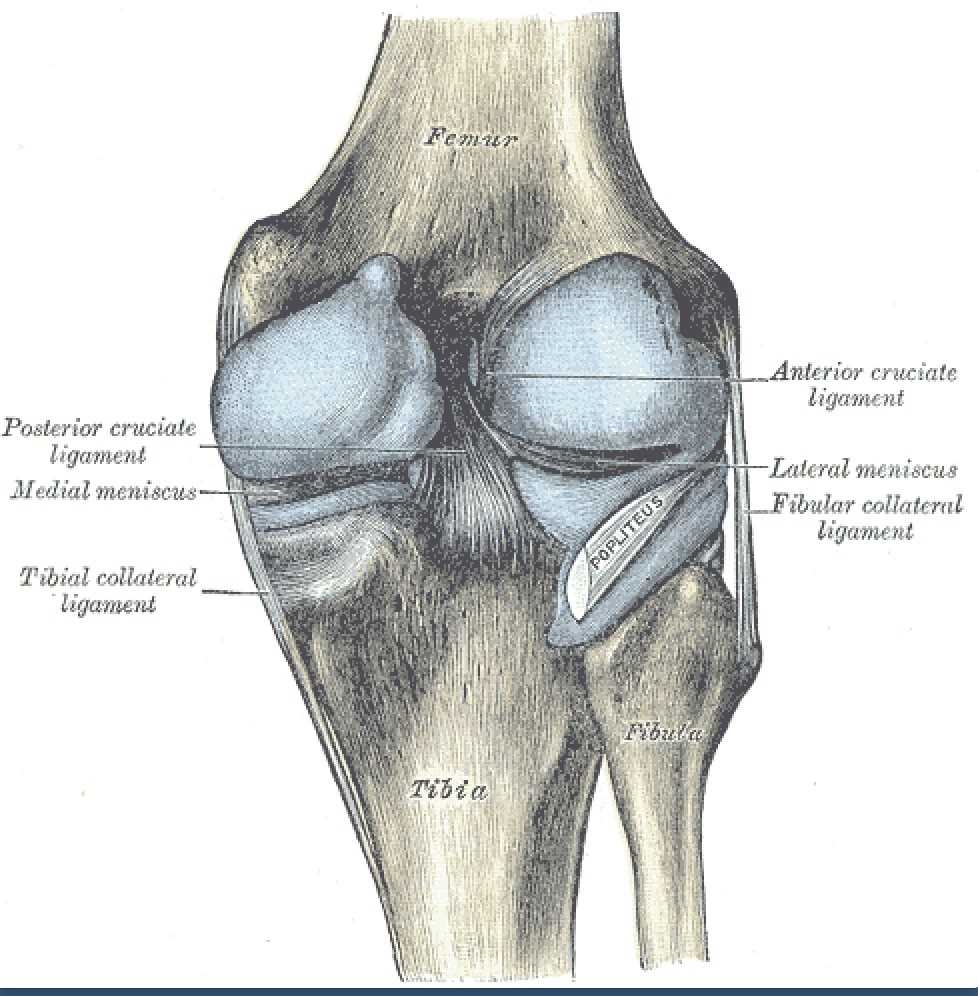
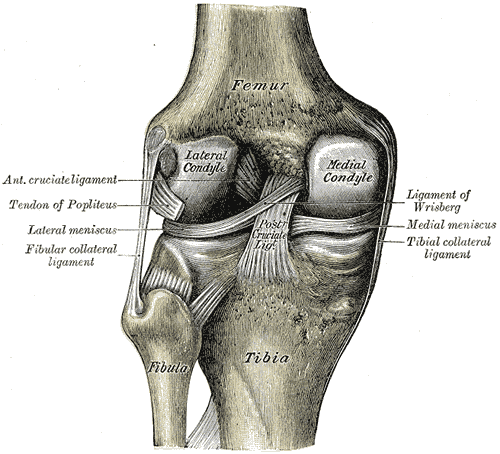
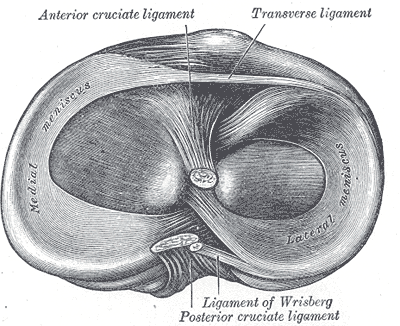
Intraarticular Ligaments Of The Right Knee, Anterior View. Shown in this illustration are the anterior cruciate and posterior cruciate ligaments and the medial and lateral menisci. The image also shows the lateral and medial femoral condyles, tibia, fibula, and anterior superior tibiofibular ligament.
Gray's Anatomy
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Duthon VB, Barea C, Abrassart S, Fasel JH, Fritschy D, Ménétrey J. Anatomy of the anterior cruciate ligament. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2006 Mar:14(3):204-13 [PubMed PMID: 16235056]
Markatos K, Kaseta MK, Lallos SN, Korres DS, Efstathopoulos N. The anatomy of the ACL and its importance in ACL reconstruction. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2013 Oct:23(7):747-52. doi: 10.1007/s00590-012-1079-8. Epub 2012 Sep 22 [PubMed PMID: 23412211]
Arnoczky SP. Anatomy of the anterior cruciate ligament. Clinical orthopaedics and related research. 1983 Jan-Feb:(172):19-25 [PubMed PMID: 6821989]
Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clinical orthopaedics and related research. 1975 Jan-Feb:(106):216-31 [PubMed PMID: 1126079]
Ellison AE,Berg EE, Embryology, anatomy, and function of the anterior cruciate ligament. The Orthopedic clinics of North America. 1985 Jan; [PubMed PMID: 3969275]
Smith BA, Livesay GA, Woo SL. Biology and biomechanics of the anterior cruciate ligament. Clinics in sports medicine. 1993 Oct:12(4):637-70 [PubMed PMID: 8261518]
Level 3 (low-level) evidenceDienst M, Burks RT, Greis PE. Anatomy and biomechanics of the anterior cruciate ligament. The Orthopedic clinics of North America. 2002 Oct:33(4):605-20, v [PubMed PMID: 12528904]
Yahagi Y, Horaguchi T, Iriuchishima T, Suruga M, Iwama G, Aizawa S. Correlation between the mid-substance cross-sectional anterior cruciate ligament size and the knee osseous morphology. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2020 Feb:30(2):291-296. doi: 10.1007/s00590-019-02552-x. Epub 2019 Sep 24 [PubMed PMID: 31552484]
Level 2 (mid-level) evidenceMarkolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee--the contributions of the supporting structures. A quantitative in vitro study. The Journal of bone and joint surgery. American volume. 1976 Jul:58(5):583-94 [PubMed PMID: 946969]
Beynnon BD, Fleming BC, Labovitch R, Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002 Mar:20(2):332-7 [PubMed PMID: 11918313]
Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. The American journal of sports medicine. 2007 Feb:35(2):223-7 [PubMed PMID: 17158275]
Domnick C, Raschke MJ, Herbort M. Biomechanics of the anterior cruciate ligament: Physiology, rupture and reconstruction techniques. World journal of orthopedics. 2016 Feb 18:7(2):82-93. doi: 10.5312/wjo.v7.i2.82. Epub 2016 Feb 18 [PubMed PMID: 26925379]
Herbort M, Lenschow S, Fu FH, Petersen W, Zantop T. ACL mismatch reconstructions: influence of different tunnel placement strategies in single-bundle ACL reconstructions on the knee kinematics. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2010 Nov:18(11):1551-8. doi: 10.1007/s00167-010-1163-8. Epub 2010 May 12 [PubMed PMID: 20461359]
Gabriel MT, Wong EK, Woo SL, Yagi M, Debski RE. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2004 Jan:22(1):85-9 [PubMed PMID: 14656664]
Fleming BC, Renstrom PA, Beynnon BD, Engstrom B, Peura GD, Badger GJ, Johnson RJ. The effect of weightbearing and external loading on anterior cruciate ligament strain. Journal of biomechanics. 2001 Feb:34(2):163-70 [PubMed PMID: 11165279]
Beynnon BD, Johnson RJ, Fleming BC, Peura GD, Renstrom PA, Nichols CE, Pope MH. The effect of functional knee bracing on the anterior cruciate ligament in the weightbearing and nonweightbearing knee. The American journal of sports medicine. 1997 May-Jun:25(3):353-9 [PubMed PMID: 9167816]
Gardner E, O'Rahilly R. The early development of the knee joint in staged human embryos. Journal of anatomy. 1968 Jan:102(Pt 2):289-99 [PubMed PMID: 5643844]
Level 3 (low-level) evidencede Carvalho RT, Ramos LA, Novaretti JV, Ribeiro LM, Szeles PR, Ingham SJ, Abdalla RJ. Relationship Between the Middle Genicular Artery and the Posterior Structures of the Knee: A Cadaveric Study. Orthopaedic journal of sports medicine. 2016 Dec:4(12):2325967116673579. doi: 10.1177/2325967116673579. Epub 2016 Dec 9 [PubMed PMID: 28050573]
Salaria H, Atkinson R. Anatomic study of the middle genicular artery. Journal of orthopaedic surgery (Hong Kong). 2008 Apr:16(1):47-9 [PubMed PMID: 18453659]
Scapinelli R. Vascular anatomy of the human cruciate ligaments and surrounding structures. Clinical anatomy (New York, N.Y.). 1997:10(3):151-62 [PubMed PMID: 9135883]
Arnoczky SP, Rubin RM, Marshall JL. Microvasculature of the cruciate ligaments and its response to injury. An experimental study in dogs. The Journal of bone and joint surgery. American volume. 1979 Dec:61(8):1221-9 [PubMed PMID: 511882]
Level 3 (low-level) evidenceKennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. The American journal of sports medicine. 1982 Nov-Dec:10(6):329-35 [PubMed PMID: 6897495]
Hogervorst T, Brand RA. Mechanoreceptors in joint function. The Journal of bone and joint surgery. American volume. 1998 Sep:80(9):1365-78 [PubMed PMID: 9759824]
Marieswaran M, Jain I, Garg B, Sharma V, Kalyanasundaram D. A Review on Biomechanics of Anterior Cruciate Ligament and Materials for Reconstruction. Applied bionics and biomechanics. 2018:2018():4657824. doi: 10.1155/2018/4657824. Epub 2018 May 13 [PubMed PMID: 29861784]
Anderson MJ, Browning WM 3rd, Urband CE, Kluczynski MA, Bisson LJ. A Systematic Summary of Systematic Reviews on the Topic of the Anterior Cruciate Ligament. Orthopaedic journal of sports medicine. 2016 Mar:4(3):2325967116634074. doi: 10.1177/2325967116634074. Epub 2016 Mar 15 [PubMed PMID: 27047983]
Level 1 (high-level) evidenceHewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. The American journal of sports medicine. 2006 Feb:34(2):299-311 [PubMed PMID: 16423913]
Renstrom P, Ljungqvist A, Arendt E, Beynnon B, Fukubayashi T, Garrett W, Georgoulis T, Hewett TE, Johnson R, Krosshaug T, Mandelbaum B, Micheli L, Myklebust G, Roos E, Roos H, Schamasch P, Shultz S, Werner S, Wojtys E, Engebretsen L. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. British journal of sports medicine. 2008 Jun:42(6):394-412. doi: 10.1136/bjsm.2008.048934. Epub [PubMed PMID: 18539658]
Geng B, Wang J, Ma JL, Zhang B, Jiang J, Tan XY, Xia YY. Narrow Intercondylar Notch and Anterior Cruciate Ligament Injury in Female Nonathletes with Knee Osteoarthritis Aged 41-65 Years in Plateau Region. Chinese medical journal. 2016 Nov 5:129(21):2540-2545. doi: 10.4103/0366-6999.192771. Epub [PubMed PMID: 27779159]
Price MJ, Tuca M, Cordasco FA, Green DW. Nonmodifiable risk factors for anterior cruciate ligament injury. Current opinion in pediatrics. 2017 Feb:29(1):55-64. doi: 10.1097/MOP.0000000000000444. Epub [PubMed PMID: 27861256]
Level 3 (low-level) evidenceBarber-Westin SD, Noyes FR, Galloway M. Jump-land characteristics and muscle strength development in young athletes: a gender comparison of 1140 athletes 9 to 17 years of age. The American journal of sports medicine. 2006 Mar:34(3):375-84 [PubMed PMID: 16282578]
Level 2 (mid-level) evidenceAlentorn-Geli E, Myer GD, Silvers HJ, Samitier G, Romero D, Lázaro-Haro C, Cugat R. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 2: a review of prevention programs aimed to modify risk factors and to reduce injury rates. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2009 Aug:17(8):859-79. doi: 10.1007/s00167-009-0823-z. Epub 2009 Jun 9 [PubMed PMID: 19506834]