Introduction
The systolic performance of the left ventricle is determined by three factors: preload, afterload, and contractility. The Frank-Starling relationship characterizes the effect of preload, often measured by left ventricular end-diastolic volume (LVEDV) or left ventricular end-diastolic pressure (LVEDP), on systolic function. It is an intrinsic property of the heart by which an increase in left ventricular end-diastolic volume leads to increased ventricular contraction. This variability means that under normal conditions, the heart can compensate for the increased delivery of blood to the left ventricle by increasing cardiac output. The exact mechanism behind this observation is not fully elucidated, but evidence shows that increasing sarcomere length in cardiomyocytes causes an increase in the active tension generated during contraction. Heart failure results when the ventricle is no longer able to provide adequate contraction for a given LVEDV. The Frank-Starling relationship is important for understanding the physiology of heart failure and developing new approaches for treating this disease.[1][2][3]
Cellular Level
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Cellular Level
Muscle consists of individual cells known as muscle fibers, which form from bundles of myofibrils. The myofibril is divided further into individual sarcomeres consisting of thick filaments and thin filaments. The thin filaments contain actin, tropomyosin, and troponin, whereas myosin forms the thick filaments, which is stabilized by a protein called titin. Muscle contraction occurs when myosin forms cross-bridges with actin and utilizes the energy provided by hydrolysis of ATP to generate active tension by pulling on actin. Tropomyosin is complexed with troponin and prevents cross-bridge formation by blocking myosin-binding sites on actin. Troponin consists of three subunits: 1) troponin T, which anchors the complex to tropomyosin, 2) troponin I, which binds to actin and blocks the myosin-binding sites, and 3) troponin C which binds to calcium. When calcium binds to troponin C, the tropomyosin-troponin unit changes its conformation leading to exposure of these binding sites, which allows contraction to occur.[4][5][6][7]
Mechanism
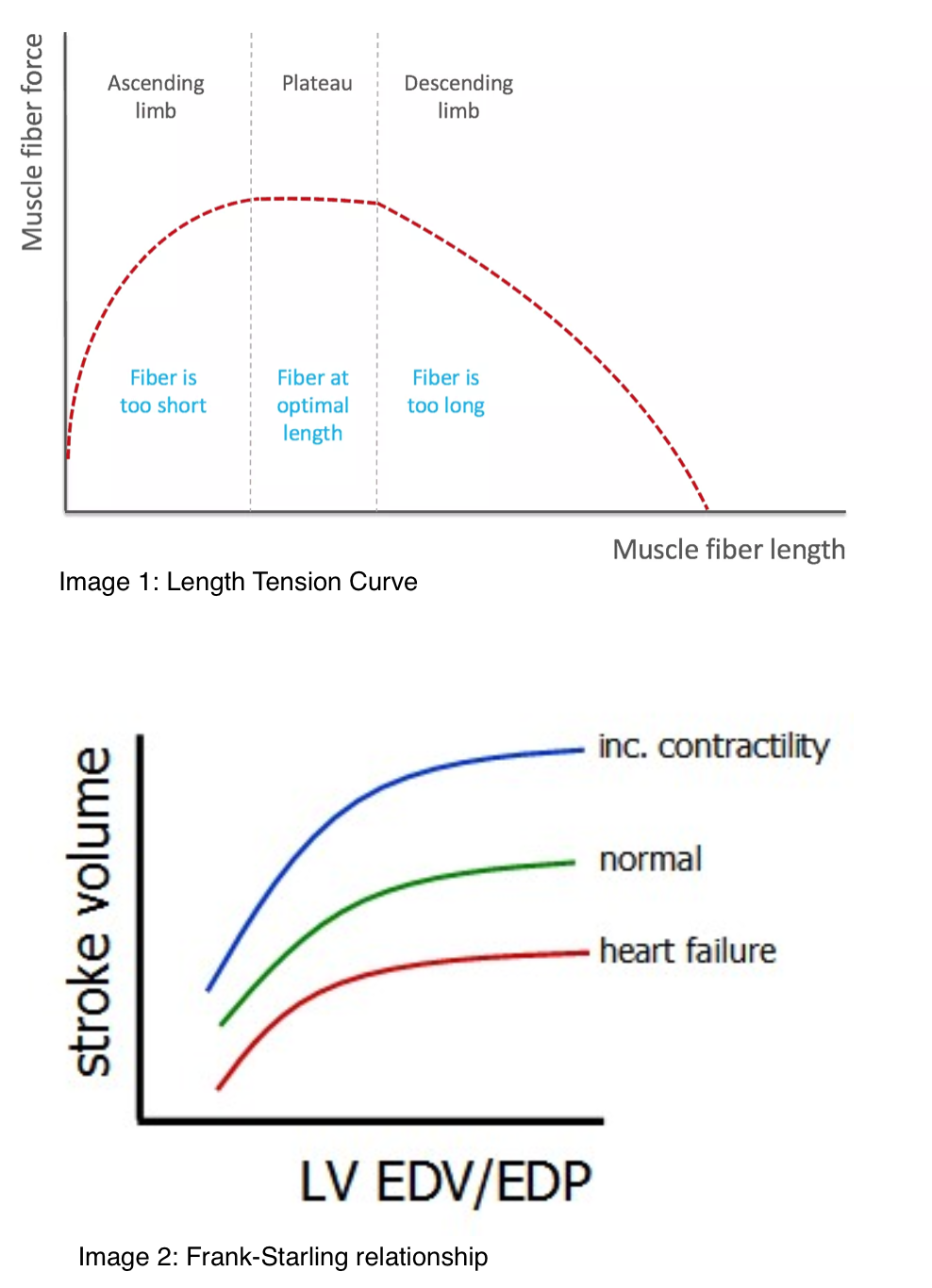
The best depiction of the mechanism of the Frank-Starling relationship is the length-tension relationship (image 1) established by Gordon et al. in 1966. Image 1 illustrates that the active tension generated by muscle contraction is a function of the passive tension provided by stretching the myofibrils.[8] It is a biphasic curve that depicts an optimal sarcomere length that achieves maximum active tension, and beyond which, a further stretch is less effective. The resting length of the skeletal muscle is near its optimal value, whereas cardiomyocytes at end-diastole are below this point. Increases in preload stretch the ventricular myocytes to a length closer to the optimum value leading to an increase in active tension. It is unclear exactly how changes in sarcomere length alter the strength of contraction. The initial line of thought was that changing the length of sarcomeres influences the overlap between thick and thin filaments, causing different amounts of cross-bridge cycling. Further studies debate these findings by showing that the amount of active tension generated could be changed while the number of cross-bridges remained constant. Decades of research have led to evidence supporting several mechanisms operating simultaneously. These include:
- Increasing sarcomere length leads to an increased affinity of troponin for calcium, which facilitates the interaction of actin and myosin.
- Stretching the myocytes leads to decreased interfilament lattice spacing, which brings actin and myosin closer together.
- Stretch causes titin to reduce lattice space and change cross-bridge orientation.
- Calcium-binding to troponin induces conformational changes in the troponin-tropomyosin complex allowing stretch-dependent activation.
- Stretching induces cooperativity in which initial cross-bridge formation potentiates further binding leading to increased active tension for any given calcium concentration.
Clinical Significance
The Frank-Starling relationship describes how the left ventricle responds to increased preload under normal conditions. Figure 2 demonstrates this in a graphical representation. The transverse axis represents preload, often measured as LVEDV or LVEDP, and the longitudinal axis is stroke volume. As passive tension due to LVEDV or LVEDP increases, the active contraction is stronger, leading to higher stroke volume and cardiac output. This increased stroke volume is represented by moving along various points on the green curve and appears similar to the length-tension relationship (image 1). Changes in contractility cause the normal curve to shift up or down, meaning there is a difference in contractility for any given preload. Of note, there is a plateau of the Frank-Starling relationship at higher amounts of preload, which might be due to increases in sarcomere length that exceed the optimal value leading to decreases in the affinity of calcium for troponin. The plateau is also attributable to the use of LVEDP instead of LVEDV in clinical practice because of its ease of measurement. In a healthy heart, LVEDP is strongly correlated with LVEDV. Decreases in compliance, often seen in heart disease, can lead to high LVEDP at lower LVEDV. As a result, there is very little stretching to produce the length-dependent activation observed in the Frank-Starling relationship.
The Frank-Starling relationship is essential in understanding the underlying pathophysiology of heart failure.[9] Heart failure is divided into diastolic and systolic dysfunction. Diastolic heart failure frequently occurs when the left ventricle has difficulty filling due to changes induced by chronic pressure overload as in hypertension or aortic stenosis. Concentric hypertrophy is an adaptation to the increased wall tension leading to the formation of new sarcomeres in parallel, causing an increase in wall thickness and a decrease in internal chamber size. Under these conditions, there is a decrease in ventricular compliance, which impairs the filling of the left ventricle. Ultimately, the reduction in preload results in a decreased stroke volume according to the principles established by the Frank-Starling relationship, which correlates with a movement towards the left along the green curve in image 2.
Systolic heart failure results from a decrease in myocardial contractility, which induces a downward and right-sided shift in the stroke volume-LVEDP curve represented by the red curve. This change means that there is a reduction of maximal cardiac output for any given preload. Initially, increases in sympathetic activity help compensate for the decline by increasing contractility and heart rate. Additionally, renal salt and water retention lead to increased preload, which increases systolic function via the Frank-Starling mechanism. However, the curve continues to shift as contractility is further diminished, eventually overwhelming the compensatory mechanisms.
Media
(Click Image to Enlarge)
References
Kuhtz-Buschbeck JP, Drake-Holland A, Noble MIM, Lohff B, Schaefer J. Rediscovery of Otto Frank's contribution to science. Journal of molecular and cellular cardiology. 2018 Jun:119():96-103. doi: 10.1016/j.yjmcc.2018.04.017. Epub 2018 May 1 [PubMed PMID: 29727607]
LaCombe P, Tariq MA, Lappin SL. Physiology, Afterload Reduction. StatPearls. 2023 Jan:(): [PubMed PMID: 29630226]
Yoshida J, Kawai M, Minai K, Ogawa K, Ogawa T, Yoshimura M. Associations between Left Ventricular Cavity Size and Cardiac Function and Overload Determined by Natriuretic Peptide Levels and a Covariance Structure Analysis. Scientific reports. 2017 May 17:7(1):2037. doi: 10.1038/s41598-017-02247-5. Epub 2017 May 17 [PubMed PMID: 28515459]
Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B, Levine BD. Impact of lifelong exercise "dose" on left ventricular compliance and distensibility. Journal of the American College of Cardiology. 2014 Sep 23:64(12):1257-66. doi: 10.1016/j.jacc.2014.03.062. Epub [PubMed PMID: 25236519]
Level 2 (mid-level) evidenceParasuraman SK, Loudon BL, Lowery C, Cameron D, Singh S, Schwarz K, Gollop ND, Rudd A, McKiddie F, Phillips JJ, Prasad SK, Wilson AM, Sen-Chowdhry S, Clark A, Vassiliou VS, Dawson DK, Frenneaux MP. Diastolic Ventricular Interaction in Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association. 2019 Apr 2:8(7):e010114. doi: 10.1161/JAHA.118.010114. Epub [PubMed PMID: 30922153]
Kumar M, Govindan S, Zhang M, Khairallah RJ, Martin JL, Sadayappan S, de Tombe PP. Cardiac Myosin-binding Protein C and Troponin-I Phosphorylation Independently Modulate Myofilament Length-dependent Activation. The Journal of biological chemistry. 2015 Dec 4:290(49):29241-9. doi: 10.1074/jbc.M115.686790. Epub 2015 Oct 9 [PubMed PMID: 26453301]
Gregory SD, Stevens M, Timms D, Pearcy M. Replication of the Frank-Starling response in a mock circulation loop. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference. 2011:2011():6825-8. doi: 10.1109/IEMBS.2011.6091683. Epub [PubMed PMID: 22255906]
Pandey A, Kraus WE, Brubaker PH, Kitzman DW. Healthy Aging and Cardiovascular Function: Invasive Hemodynamics During Rest and Exercise in 104 Healthy Volunteers. JACC. Heart failure. 2020 Feb:8(2):111-121. doi: 10.1016/j.jchf.2019.08.020. Epub 2019 Nov 6 [PubMed PMID: 31706837]
Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, Urey MA, Adams-Huet B, Levine BD. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation. 2018 Apr 10:137(15):1549-1560. doi: 10.1161/CIRCULATIONAHA.117.030617. Epub 2018 Jan 8 [PubMed PMID: 29311053]
Level 1 (high-level) evidence