Introduction
The pancreas is a composite organ, which has exocrine and endocrine functions. The endocrine portion is arranged as discrete islets of Langerhans, which are composed of five different endocrine cell types (alpha, beta, delta, epsilon, and upsilon) secreting at least five hormones including glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide, respectively.
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
Pancreatic Hormones and Their Function[1][2][3]
Insulin
Source: Beta cells of islets of the pancreas.
Synthesis: Insulin is a peptide hormone. The insulin mRNA is translated as a single-chain precursor called preproinsulin, and removal of its signal peptide during insertion into the endoplasmic reticulum generates proinsulin. Within the endoplasmic reticulum, proinsulin is exposed to several specific endopeptidases, which excise the C peptide (one of three domains of proinsulin), thereby generating the mature form of insulin. Insulin is secreted from the cell by exocytosis and diffuses into islet capillary blood. C-peptide is also secreted into the blood in a 1:1 molar ratio with insulin. Although C-peptide has no established biological action, it is used as a useful marker for insulin secretion.
Transport: insulin circulates entirely in unbound form (T1/2 = 6 min).
Main Target cells: hepatic, muscle and adipocyte cells (i.e., cells specialized for energy storage).
Mechanism of action: Insulin binds to a specific receptor tyrosine kinase on the plasma membrane and increases its activity to phosphorylate numerous regulatory enzymes and other protein substrates.
Regulation of its secretion:
Plasma glucose level is the main regulator of insulin secretion. The change in the concentration of plasma glucose that occurs in response to feeding or fasting is the main determinant of insulin secretion. Modest increases in plasma glucose level provoke a marked increase in plasma insulin concentration. Glucose is taken up by beta cells via glucose transporters (GLUT2). The subsequent metabolism of glucose increases cellular adenosine triphosphate (ATP) concentrations and closes ATP-dependent potassium (KATP) channels in the beta cell membrane, causing membrane depolarization and an influx of calcium. Increased calcium intracellular concentration results in an increase of insulin secretion.
Increased plasma amino acid and free fatty acid concentrations induce insulin secretion as well.
Glucagon is also known to be a strong insulin secretagogue.
Physiological functions:
Insulin plays an important role to keep plasma glucose value within a relatively narrow range throughout the day (glucose homeostasis). Insulin’s main actions are (1) In the liver, insulin promotes glycolysis and storage of glucose as glycogen (glycogenesis), as well as conversion of glucose to triglycerides, (2) In muscle, insulin promotes the uptake of glucose and its storage as glycogen, and (3) in adipose tissue, insulin promotes uptake of glucose and its conversion to triglycerides for storage.
Amylin (diabetes-associated peptide)
Source: Beta cells of islets of the pancreas. It is co-secreted with insulin in response to caloric intake (feeding state).
Target cells: Alpha cells of islets of pancreas and hypothalamus.
Physiological functions: it suppresses glucagon secretion from the alpha cells of the islets in the pancreas via paracrine interaction between beta cells and alpha cells. Amylin also slows gastric emptying which delays absorption of glucose from the small intestine into the circulation. Also, it stimulates the satiety center of the brain to limit food consumption.
Glucagon
Source: Alpha cells of islets of the pancreas.
Synthesis: The initial gene product is the mRNA encoding preproglucagon. A peptidase removes the signal sequence of preproglucagon during translation of the mRNA in the rough endoplasmic reticulum to yield proglucagon. Proteases in the alpha cells subsequently cleave the proglucagon into the mature glucagon molecule.
Target cells: Hepatic cells.
Mechanism of action: glucagon binds to a receptor that activates the heterotrimeric G protein Gas, which stimulates membrane-bound adenylyl cyclase. The cAMP formed by adenylyl cyclase, in turn, activates PKA, which phosphorylates numerous regulatory enzymes and other protein substrates.
Regulation of its secretion: The amino acids released by digestion of a protein meal appear to be the main determinant of glucagon secretion.
Physiological functions: Glucagon acts exclusively on the liver to antagonize insulin effects on hepatocytes. It enhances glycogenolysis and gluconeogenesis. It also promotes oxidation of fat, which can lead to the formation of ketone bodies.
Somatostatin
Source: Delta cells of the islets of the pancreas, hypothalamus and D cells of gastric glands.
Target cells: Beta cells of islets of the pancreas, somatotroph cells in the anterior pituitary gland and the G cells of the gastric glands.
Mechanism of action: Somatostatin binds to a receptor that activates the heterotrimeric inhibitory G protein, which inhibits membrane-bound adenylyl cyclase and cAMP formation.
Regulation of its secretion: Glucagon stimulates somatostatin secretion via paracrine interaction between alpha cells and delta cells of the islets of the pancreas.
Physiological functions: Somatostatin inhibits the secretion of multiple hormones, including growth hormone, insulin, glucagon, gastrin, vasoactive intestinal peptide (VIP), and thyroid-stimulating hormone.
Ghrelin
Source: Epsilon cells of the islets of the pancreas, endocrine cells in the stomach and hypothalamus.
Target cells: Beta cells of the islets of the pancreas and somatotroph cells in the anterior pituitary gland.
Physiological functions: ghrelin inhibits the secretion of insulin from Beta cells of the islets of the pancreas via paracrine interaction between delta cells and beta cells of the islets of the pancreas. It also stimulates appetite and growth hormone secretion.
Pancreatic Polypeptide (PP)
Pancreatic polypeptide is secreted from upsilon (F) cells of the islets of the pancreas. Dietary intake of nutrients alters the secretion of the pancreatic polypeptide. Its function is not decidedly understood yet.
Paracrine Interaction Between Pancreatic Endocrine Cells
Insulin secreted by beta cells acts as a prime hormone of glucose homeostasis. Insulin and amylin inhibit glucagon secretion by alpha cells. Whereas glucagon activates insulin and somatostatin secretion, somatostatin secreted by delta cells and ghrelin by epsilon cells inhibit insulin secretion.
Clinical Significance
Diabetes Mellitus (DM) is a chronic disease that occurs when the pancreas cannot produce enough insulin, or the body cannot effectively utilize insulin, which results in high glucose plasma level (hyperglycemia) causing tissue damage over time. There are two common types of DM that account for the majority of cases: type 1 and type 2.[4][5][6]
Type 1 DM
It is a chronic autoimmune disease in which the beta cells of islets of the pancreas are destroyed resulting in insulin deficiency.
Pathophysiology: It is not entirely understood yet, but it is caused by a combination of events in genetically susceptible individuals. Three mechanisms lead to islet cell destruction: genetic susceptibility, autoimmunity, and environmental insult(s). A virus or allergen (environmental insults) in genetically susceptible individuals induces the production of autoantibodies to Beta cells of the islets of the pancreas. This autoimmune reaction creates autoreactive T cells that destroy beta-islet cells and cause loss of insulin secretion.
Diagnosis: The serum autoantibodies serve as a marker. The autoantibodies are typically present years before the diagnosis of T1DM is made because clinical manifestations appear after 80% of beta-islet cells have been destroyed.[7]
Type 2 DM
It is a progressive disease that develops due to a continued decline in beta-cell function and/or due to a defect in insulin sensitivity that causes hyperglycemia. The development and rate of progression of T2D are influenced by both genetic and environmental factors, such as obesity and physical inactivity.
Pathophysiology: Beta-cell dysfunction manifests in different ways: (1) reductions in insulin release, (2) changes in pulsatile insulin secretion, (3) an abnormality in the efficiency of proinsulin to insulin conversion, and (4) reduces the release of amylin. Insulin resistance is present in most patients with T2D. Insulin resistance is characterized by higher than expected plasma glucose level with the prevailing plasma insulin secretion. In patients with T2D insulin, stimulation fails to induce normal GLUT4 protein translocation to the sarcolemma in skeletal muscle membrane. Also, excessive production of free fatty acids and overexpression of TNF-alpha by adipocytes have been proposed as mechanisms for the development of insulin resistance.[8]
Media
(Click Image to Enlarge)
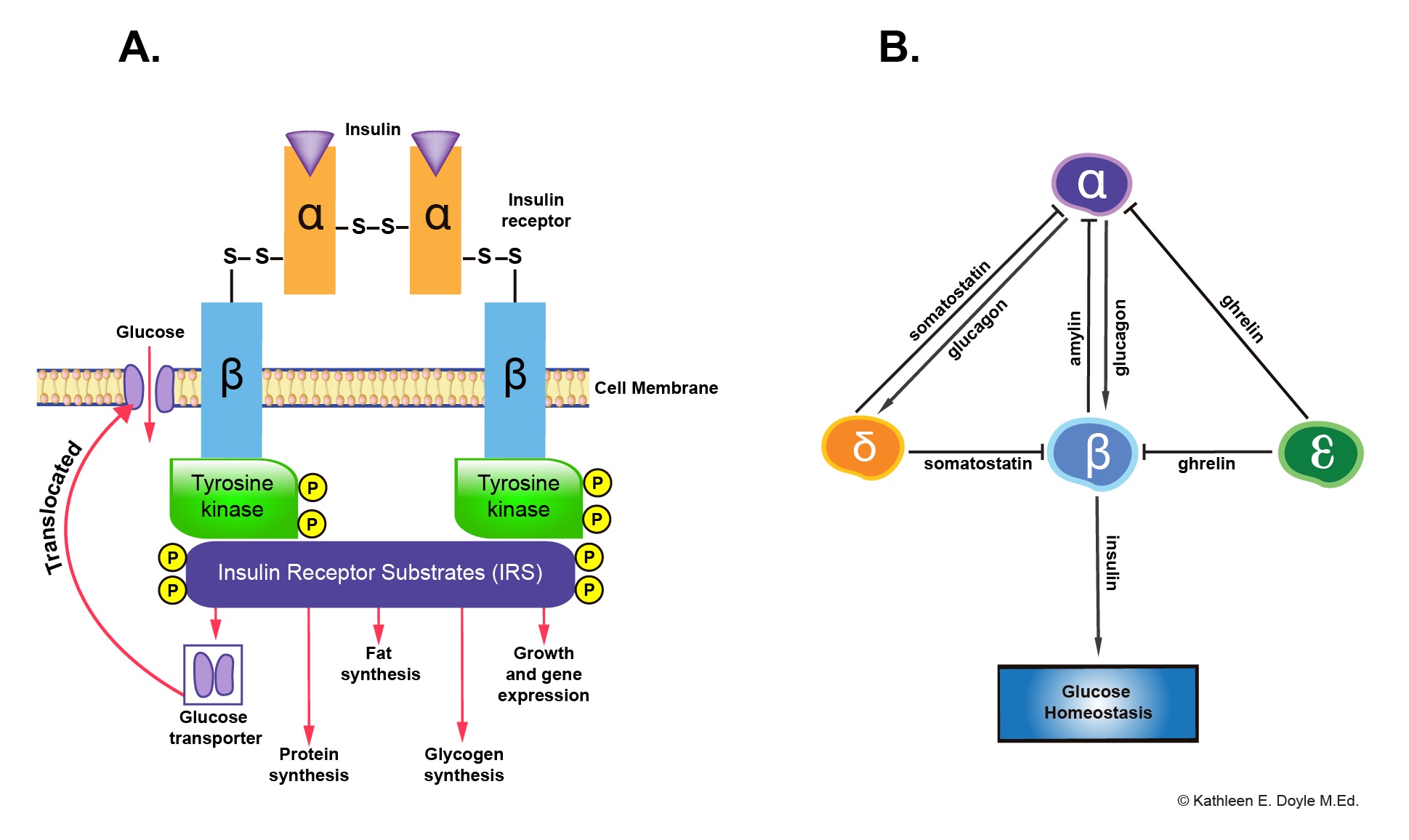
Insulin Receptor and Paracrine Interaction in Pancreatic Islet Cells. (A) Insulin receptor and subsequent functions, (B) Paracrine interaction among different types of pancreatic islet cells. Contributed by Kathleen E Doyle MEd, Instructional Designer at the Oakland University William Beaumont School of Medicine.
References
O'Toole TJ, Sharma S. Physiology, Somatostatin. StatPearls. 2023 Jan:(): [PubMed PMID: 30855911]
Capurso G, Traini M, Piciucchi M, Signoretti M, Arcidiacono PG. Exocrine pancreatic insufficiency: prevalence, diagnosis, and management. Clinical and experimental gastroenterology. 2019:12():129-139. doi: 10.2147/CEG.S168266. Epub 2019 Mar 21 [PubMed PMID: 30962702]
Matschinsky FM, Wilson DF. The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans. Frontiers in physiology. 2019:10():148. doi: 10.3389/fphys.2019.00148. Epub 2019 Mar 6 [PubMed PMID: 30949058]
Level 3 (low-level) evidenceAllen N, Gupta A. Current Diabetes Technology: Striving for the Artificial Pancreas. Diagnostics (Basel, Switzerland). 2019 Mar 15:9(1):. doi: 10.3390/diagnostics9010031. Epub 2019 Mar 15 [PubMed PMID: 30875898]
Vettoretti M, Facchinetti A. Combining continuous glucose monitoring and insulin pumps to automatically tune the basal insulin infusion in diabetes therapy: a review. Biomedical engineering online. 2019 Mar 29:18(1):37. doi: 10.1186/s12938-019-0658-x. Epub 2019 Mar 29 [PubMed PMID: 30922295]
Guo YY, Li HX, Zhang Y, He WH. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discovery medicine. 2019 Feb:27(147):101-109 [PubMed PMID: 30939294]
Lovic D,Piperidou A,Zografou I,Grassos H,Pittaras A,Manolis A, The Growing Epidemic of Diabetes Mellitus. Current vascular pharmacology. 2020 [PubMed PMID: 30961501]
Heo CU, Choi CI. Current Progress in Pharmacogenetics of Second-Line Antidiabetic Medications: Towards Precision Medicine for Type 2 Diabetes. Journal of clinical medicine. 2019 Mar 21:8(3):. doi: 10.3390/jcm8030393. Epub 2019 Mar 21 [PubMed PMID: 30901912]