Introduction
As the largest of the cranial nerves, the trigeminal nerve is responsible for the primary sensory input from the head and neck as well as providing motor innervation to the muscles of mastication. The trigeminal nerve innervates key vascular structures such as the brainstem, the cavernous sinus, and peripheral divisions. With such an extensive distribution within the head and neck, various lesions may contribute to trigeminal neuralgia or dysfunction. The goal of this activity is to review the neuroanatomy of the trigeminal nerve and cover the reflex tests employed to distinguish between lesions of the trigeminal nerve.
The trigeminal nerve is the fifth cranial nerve (CN V) and the largest of the paired cranial nerves. It is a mixed nerve that partially innervates the craniofacial region along with the facial nerve. The fifth cranial nerve contains three terminal branches that innervate the skin of the face and neck, mucous membranes and paranasal sinuses of the face, the corneas, and the muscles of mastication. A trigeminal nerve reflex is a natural response to stimuli indicating the proper functioning of the nerve. Each area innervated by the trigeminal nerve can undergo assessment through the presence and strength of a trigeminal nerve reflex.[1][2][3][4]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Structure and Function:
The complexity of its neuroanatomy matches the size of the CN V. The trigeminal nerve fibers arise from the trigeminal nerve nucleus, which extends from the midbrain to the upper cervical spinal cord. The trigeminal nerve nucleus is the largest of all cranial nerve nuclei. It divides into three main portions: (1) the mesencephalic trigeminal nucleus, which receives mechanoreceptor fibers and proprioceptive information from the mandibles and teeth, (2) the main trigeminal nucleus, which receives the majority of the touch and position fibers, and the (3) spinal trigeminal nucleus which primarily receives temperature and pain stimuli.[2][1][5][6]
The sensory nuclei merge at the level of the pons to form a sensory root. The motor nuclei continue inferiorly to the sensory nuclei to form a motor root. The sensory root then expands into the trigeminal ganglion at the level of the middle cranial fossa or Meckel’s cave. It is within this fossa that the nerves combine to form the semilunar ganglion. The position of the superior petrosal sinus is located medially to these structures. The basilar venous plexus and ventral aspects of the pons and brainstem are medial to the Meckel’s cave.[2][7][8]
Upon exiting the trigeminal ganglion, the fibers of CNV take a superomedial route toward the pons. At this point, both divisions of CN V pierce the lateral surface of the pons. Within the pons, the sensory fibers divide equally into ascending and descending divisions. Ascending fibers take a superior course towards the mesencephalic nucleus while the descending fibers will take a route toward the spinal trigeminal nucleus. Remaining sensory fibers follow a dorsomedial course toward the main sensory nucleus. Simultaneous to the dorsomedial travel of the sensory fibers toward the sensory nucleus, the remaining motor fibers will follow a similar path toward their respective motor nuclei.[2][5][9]
The bilateral pair of the mesencephalic nucleus consists of a collection of unipolar neurons extending from the main sensory nucleus within the pons. It is primarily involved in processing information related to proprioception. Myelinated axons exit the mesencephalic tract and divide into central and peripheral divisions. Central branches conduct impulses from the neuromuscular spindles of the bite-force reflex arcs within the muscles of mastication toward the motor neuron of CN V. Several other central branch axons integrate with the sensory aspect of CN V via the reticular formation or migrate toward the cerebellum via the superior cerebellar peduncle. The extensive networking of the trigeminal nerve motor and sensory divisions contribute to the regulation of the masticatory stretch muscles and the process of deglutition. The mesencephalic nucleus peripheral branches travel as fibers via the maxillary branch of the CN V in the upper jaw and the mandibular branch of the CN V in the lower jaw.[1][2][7]
Lateral to the motor nucleus of CN V lies the main sensory nucleus inside of the dorsal portion of the pontine tegmentum. Fibers derived from the mesencephalic nucleus and afferent spinal axons feed proprioceptive impulses toward the main sensory nucleus. Various larger fibers also convey discriminative touch and light touch impulses toward the main sensory nucleus. Neurons within the main sensory nucleus from the trigeminal nuclei and the pontine coalesce as the ventral trigeminothalamic tract. Other fibers within the main nucleus travel toward the thalamus forming the dorsal trigeminothalamic tract. The ventral and dorsal tracts coalesce in the area of the pons adjacent to the midbrain and are collectively known as the trigeminal lemniscus tract. Fibers within this tract access the ventral posterior medial aspect of the thalamus and ascend into an internal capsule toward the postcentral gyrus in which sensory information gets processed.[2][10][7]
The CN V motor nucleus is a conglomerate of multipolar neurons medially situated to the pontine trigeminal nucleus. The tracts of the motor nucleus innervate certain muscles within the first pharyngeal arch, including the pterygoid muscles, the masseter, and temporalis muscles. The motor nucleus is deep to the lateral rhomboid fossa in the upper division of the pontine tegmentum. The motor axons eventually merge with the mandibular V3 division of CN V. The motor nucleus is regulated through sensory input from the cerebral cortices bilaterally, as well as via the afferent fibers of the mesencephalic nuclei.[10][11][12]
Spanning the full length of the medulla oblongata to the proximal spinal cord at the level of the second or third cervical segment is the spinal nucleus of CN V. The spinal trigeminal nucleus divides into three smaller nuclei based on location. Most proximal to caudal, these are the pars oralis, pars interpolaris, and pars caudalis. The spinal trigeminal tract feeds afferent fibers, while non-myelinated central processes from the ganglion feed into the spinal nucleus. These fibers descend by way of the superior pontine sulcus after passing through the pons and carry sensory information from the three CN V divisions. The fibers of the trigeminal tract have a specific somatotopic organization in which fibers of the CN V2 run centrally and are flanked ventrolaterally and dorsomedially by the ophthalmic and mandibular divisions respectively. These tracts also contain general somatic afferent fibers arising from other cranial nerves like the vagus (CNX), glossopharyngeal (CN IX), and facial (CN VII) nerves.[13][1][10][5]
Nerves
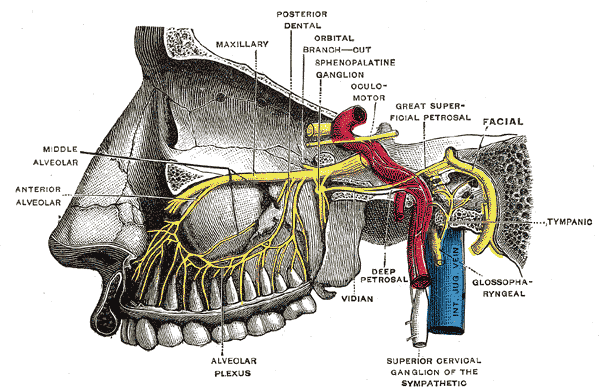
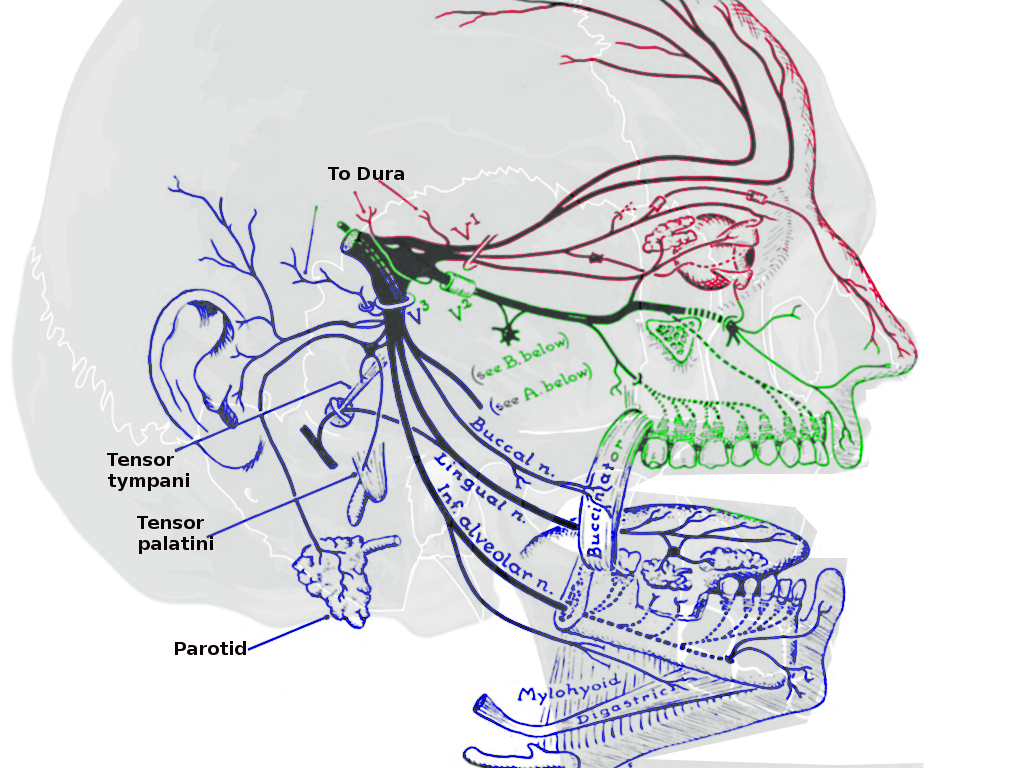
Trigeminal Nerve Divisions
The sensory aspect of CN V provides the primary innervation to the brainstem, skin of the face, paranasal sinuses, cornea, facial and masticatory muscles, the temporomandibular joint (TMJ), most of the dura mater and cerebral arteries. The brachial motor nerves of CN V innervate the masticatory muscles (including the masseter, lateral and medial pterygoids, and the temporalis), the mylohyoid, tensor veli palatini, and the tensor tympani. The CN V further divides into three main branches: the ophthalmic branch (V1), the maxillary branch (V2), and the mandibular branch (V3). Although there are no innate parasympathetic nerve fibers within CN V, the main branches are associated with several parasympathetic ganglia throughout the course of innervation.[1][11][5][7]
The ophthalmic branch of CN V additionally divides into three terminal branches: the frontal, lacrimal, and nasociliary. These terminal branches innervate the frontonasal prominence derivatives and mucous membranes of the cornea, dorsum of the nose, forehead, and scalp, the frontal and ethmoid sinus, and the upper eyelid along with its conjunctiva. The lacrimal gland receives innervation from the lacrimal branch of CN V as well as parasympathetic innervation from the postganglionic fibers from the pterygopalatine ganglion derived from CN VII.[3][14][15]
The maxillary branch of CN V is additionally divided into fourteen terminal branches innervating the mucous membranes and sinuses of derivatives of the maxillary prominence of the first pharyngeal arch. These structures include the superior palate, upper lip, upper molar, and associated gingiva, nasal cavity and lateral nose, lower eyelid and its conjunctiva, and the maxillary sinus. Similar to the ophthalmic branch, the postganglionic fibers from the pterygopalatine ganglion of CN VII join with V2 to supply parasympathetic innervation to the lacrimal gland. The nasal glands also receive parasympathetic innervation from fibers that travel alongside the nasopalatine and greater palatine nerve branches of V2.[1][2][11][16]
The mandibular branch of CN V is further subdivides into four terminal branches within the infra-temporal fossa: alveolar, auriculotemporal, buccal, and lingual nerves. These nerves supply sensory innervation to the external ear, chin, lower lip, anterior two-thirds of the tongue, lower molars, and associated gingiva, and the mucous membranes of the floor of the oral cavity. The mandibular branch is the only branch of the CN V with motor innervation, as well. The supplied structures include the muscles of mastication (medial and lateral pterygoids, masseter, temporalis), tensor veli palatini, tensor tympani, and the suprahyoid muscles. The submandibular and sublingual glands receive parasympathetic innervation via the post-ganglionic nerve fibers from CN VII that travel along with the lingual nerve. The parotid gland also receives parasympathetic innervation via fibers of CN IX that travel parallel to the auriculotemporal branch of V3.[13][11]
Clinical Significance
Examination of Trigeminal Nerve Reflexes
As the trigeminal nerve supplies sensory and motor innervation to a plethora of features, clinical examination of CN V should evaluate the response of each structure. The integrity of these modalities may be examined through the evaluation of trigeminal nerve reflexes such as the corneal and jaw jerk reflexes. The presence of the reflex represents the natural response of a normally functioning CN V tract. The absence of or variation in the strength of the reflex assists in localizing the lesion and assessing the severity of the abnormality within the CN V tract.[17][13][3][1][18]
Sensory Examination
Nociception and light touch are the principal sensory modalities tested during the assessment of CN V sensory reflexes. Light touch testing traditionally uses the cotton wisp touch test. A wisp of cotton lightly touches an area of exposed skin not innervated by CN V. This serves as a reference point, assuming that there are no sensory deficits in that area. The examiner instructs the patient to close their eyes and say "yes" each time they feel a touch. Once the patient has closed their eyes, the examiner proceeds to lightly touch areas of the skin innervated by CN V. This test is performed bilaterally to determine if a deficit is unilateral or bilateral. In the assessment of nociception, the examiner performs similar steps to the cotton wisp touch test, replacing the cotton wisp with a sharp neurotip.[17][3][18][14]
Corneal Reflex
The corneal reflex is the involuntary bilateral blinking of the eyelids stimulated by tactile, painful, or thermal stimulation of the cornea. In the context of this reflex, the ophthalmic nerve acts as the afferent limb detecting the stimuli while the facial nerve is the efferent limb, responding with the contraction of the orbicularis oculi muscle.[3][18]
The blink reflex subdivides into two stages: early and late. Early-stage reflexes involve A-beta fibers, which initiate the movement of the eyelid on the ipsilateral side. Late-stage reflexes are largely modulated by the secondary motor system and stimulate the facial nerves bilaterally such that both eyes blink. The absence of such a reflex is indicative of damage to the trigeminal/ophthalmic nerve or the facial nerve. Damage can occur within the central or peripheral nervous system at any point. Peripheral damage causes ipsilateral reflex deficits, while central damage tends to be associated with bilateral deficits.[3][14][15]
The corneal blink reflex is also under the influence of the presence of disorders and environmental factors that alter the anatomy of the trigeminal nerve within the cornea. Glaucoma is a disorder that increases the intraocular pressure within the eye, causing the trigeminal nerves to bend. The bends within the nerve correlate with a decreased corneal blink reflex sensitivity and increased ocular pathology. Diabetes also contributes to a reduced late-phase corneal reflex sensitivity from the decreased density of nerve branches and plexuses secondary to diabetic peripheral neuropathy.[3][18][19]
Jaw Jerk Reflex
The jaw jerk reflex is a stretch reflex used to assess the integrity of the upper motor neurons projecting into the trigeminal motor nucleus. The examiner places a finger between the patient’s lower lip and chin while the mouth is slightly open, and then taps the finger at a downward angle. The masseter muscles will jerk the mandible upwards in response. This reflex is normally slight or absent, but those with upper motor neuron lesions demonstrate an exaggerated jaw jerk reflex.[1][15][10]
Lacrimal Reflex
Within the lacrimal reflex arc, irritation of the cornea and conjunctiva stimulate the production of tears. The ciliary fibers of the nasociliary branch of V1 in the trigeminal nerve act as the afferent limb of the reflex arc. The facial nerve (CN VII) and the superior salivary nucleus make up the central nervous system (CNS) component of the reflex arc. Preganglionic parasympathetic secretomotor fibers travel parallel to the lacrimal branch of V1 and the zygomatic branch of V2. A lesion to any component within the reflex arc may contribute to an abnormal reflex or watering of the eyes without an appropriate stimulus.[20][11][20]
Motor Examination
Motor examination of the trigeminal nerve involves testing the superficial muscles of mastication: the temporalis and master muscles. This assessment occurs through palpation of the masseter and temporalis while the patient clenches their teeth, which allows the examiner to assess muscle mass. The strength of the muscles may be further assessed by placing the fingers of the examining hand beneath the chin and asking the patient to open the mouth and resist closure. Evaluation of the function of the motor nucleus is possible by asking the patient to open their mouth and deviate the jaw from left to right. Unintentional jaw deviation toward one side indicates a lesion to the trigeminal nerve nuclei innervating the pterygoid muscle on the affected side.[17][21][10]
Media
(Click Image to Enlarge)
(Click Video to Play)
(Click Image to Enlarge)
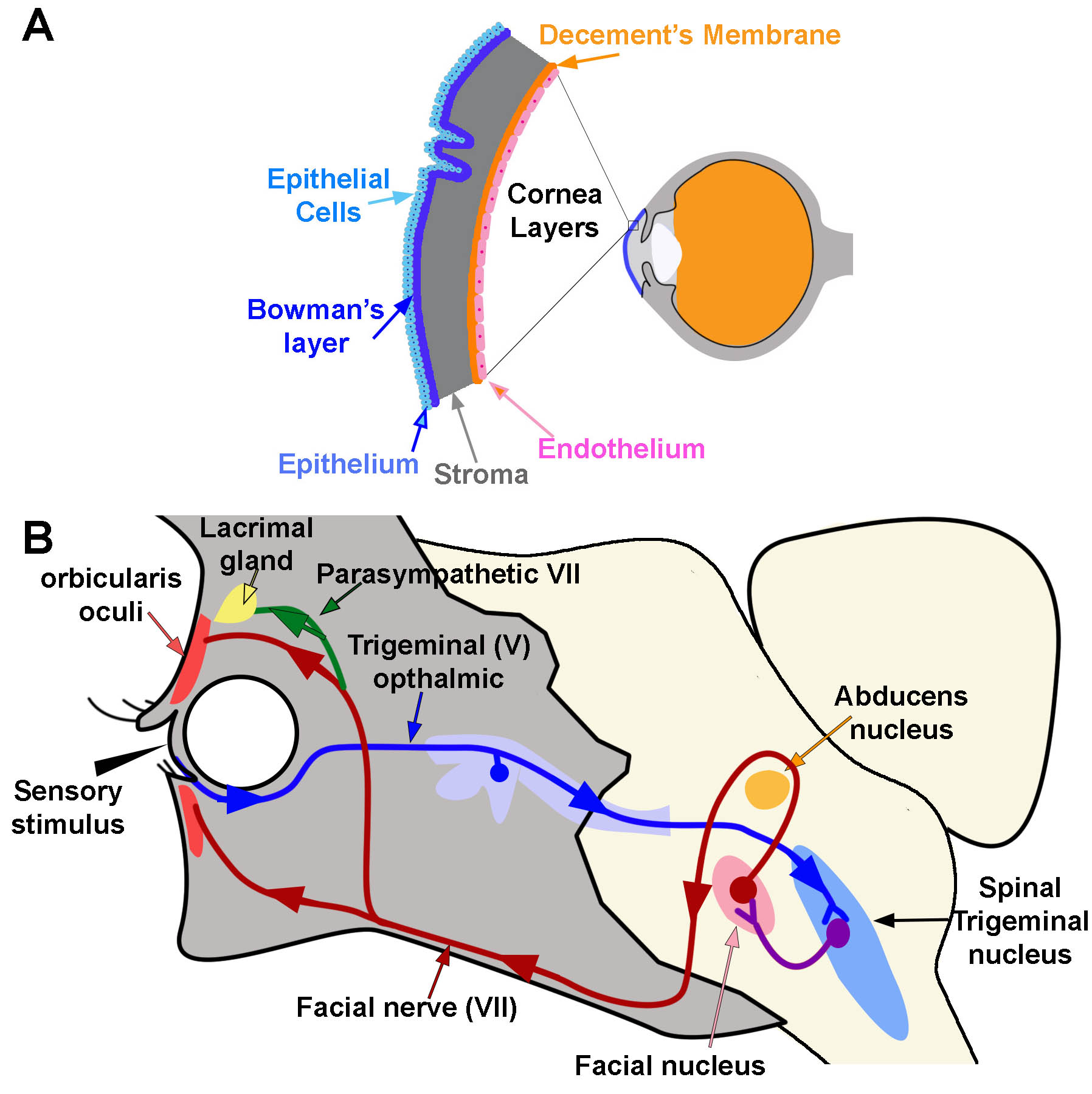
Corneal Reflex. A) Diagram of the location and layers of the cornea. B) Corneal Reflex. The blink reflex circuit is shown in blue, purple, and red. Arrows indicate the direction of flow. The tear production reflex utilizes the same pathways as the blink reflex. However it activates the lacrimal gland via parasympathetic branches (green line) Contributed and Created by Dr. Diana Peterson
References
Liang HC, Zhang JD, Luo PF, Qiao Y, Su AL, Zhang T, Zhu HN. Is Marcus Gunn jaw winking a primitive reflex? Rat neuroanatomy. International journal of ophthalmology. 2018:11(3):382-388. doi: 10.18240/ijo.2018.03.06. Epub 2018 Mar 18 [PubMed PMID: 29600170]
Level 2 (mid-level) evidenceJoo W, Yoshioka F, Funaki T, Mizokami K, Rhoton AL Jr. Microsurgical anatomy of the trigeminal nerve. Clinical anatomy (New York, N.Y.). 2014 Jan:27(1):61-88. doi: 10.1002/ca.22330. Epub 2013 Dec 9 [PubMed PMID: 24323792]
Costa YM, Baad-Hansen L, Bonjardim LR, Conti PCR, Svensson P. Reliability of the nociceptive blink reflex evoked by electrical stimulation of the trigeminal nerve in humans. Clinical oral investigations. 2017 Nov:21(8):2453-2463. doi: 10.1007/s00784-016-2042-6. Epub 2017 Jan 10 [PubMed PMID: 28074292]
Manning KA, Evinger C. Different forms of blinks and their two-stage control. Experimental brain research. 1986:64(3):579-88 [PubMed PMID: 3803493]
Level 3 (low-level) evidencePatel NM, Jozsa F, M Das J. Neuroanatomy, Spinal Trigeminal Nucleus. StatPearls. 2023 Jan:(): [PubMed PMID: 30969551]
Sonne J, Lopez-Ojeda W. Neuroanatomy, Cranial Nerve. StatPearls. 2023 Jan:(): [PubMed PMID: 29261885]
Price S, Daly DT. Neuroanatomy, Trigeminal Nucleus. StatPearls. 2024 Jan:(): [PubMed PMID: 30969645]
Djalilian HR, Thakkar KH, Hamidi S, Benson AG, Mafee MF. A study of middle cranial fossa anatomy and anatomic variations. Ear, nose, & throat journal. 2007 Aug:86(8):474, 476-81 [PubMed PMID: 17915670]
Sanders RD. The Trigeminal (V) and Facial (VII) Cranial Nerves: Head and Face Sensation and Movement. Psychiatry (Edgmont (Pa. : Township)). 2010 Jan:7(1):13-6 [PubMed PMID: 20386632]
Walker HK, Hall WD, Hurst JW, Walker HK. Cranial Nerve V: The Trigeminal Nerve. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:(): [PubMed PMID: 21250225]
Shafique S, M Das J. Anatomy, Head and Neck, Maxillary Nerve. StatPearls. 2023 Jan:(): [PubMed PMID: 31194417]
Borges A. Trigeminal neuralgia and facial nerve paralysis. European radiology. 2005 Mar:15(3):511-33 [PubMed PMID: 15690205]
Möller M, Schroeder CF, May A. Vagus nerve stimulation modulates the cranial trigeminal autonomic reflex. Annals of neurology. 2018 Dec:84(6):886-892. doi: 10.1002/ana.25366. Epub 2018 Nov 30 [PubMed PMID: 30362165]
Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Survey of ophthalmology. 2014 May-Jun:59(3):263-85. doi: 10.1016/j.survophthal.2013.09.002. Epub 2014 Jan 23 [PubMed PMID: 24461367]
Level 3 (low-level) evidenceKimura J, Powers JM, Van Allen MW. Reflex response of orbicularis oculi muscle to supraorbital nerve stimulation. Study in normal subjects and in peripheral facial paresis. Archives of neurology. 1969 Aug:21(2):193-9 [PubMed PMID: 5797352]
Huff T, Weisbrod LJ, Daly DT. Neuroanatomy, Cranial Nerve 5 (Trigeminal). StatPearls. 2023 Jan:(): [PubMed PMID: 29489263]
Hughes MA, Frederickson AM, Branstetter BF, Zhu X, Sekula RF Jr. MRI of the Trigeminal Nerve in Patients With Trigeminal Neuralgia Secondary to Vascular Compression. AJR. American journal of roentgenology. 2016 Mar:206(3):595-600. doi: 10.2214/AJR.14.14156. Epub [PubMed PMID: 26901017]
Casale R, Frazzitta G, Fundarò C, Balbi P, Del Rosso A, Bertinotti L, Matucci-Cerinic M. Blink reflex discloses CNS dysfunction in neurologically asymptomatic patients with systemic sclerosis. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2004 Aug:115(8):1917-20 [PubMed PMID: 15261870]
Kallinikos P, Berhanu M, O'Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Investigative ophthalmology & visual science. 2004 Feb:45(2):418-22 [PubMed PMID: 14744880]
Modi P, Arsiwalla T. Crocodile Tears Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 30247828]
Cruccu G, Deuschl G. The clinical use of brainstem reflexes and hand-muscle reflexes. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2000 Mar:111(3):371-87 [PubMed PMID: 10699396]