Introduction
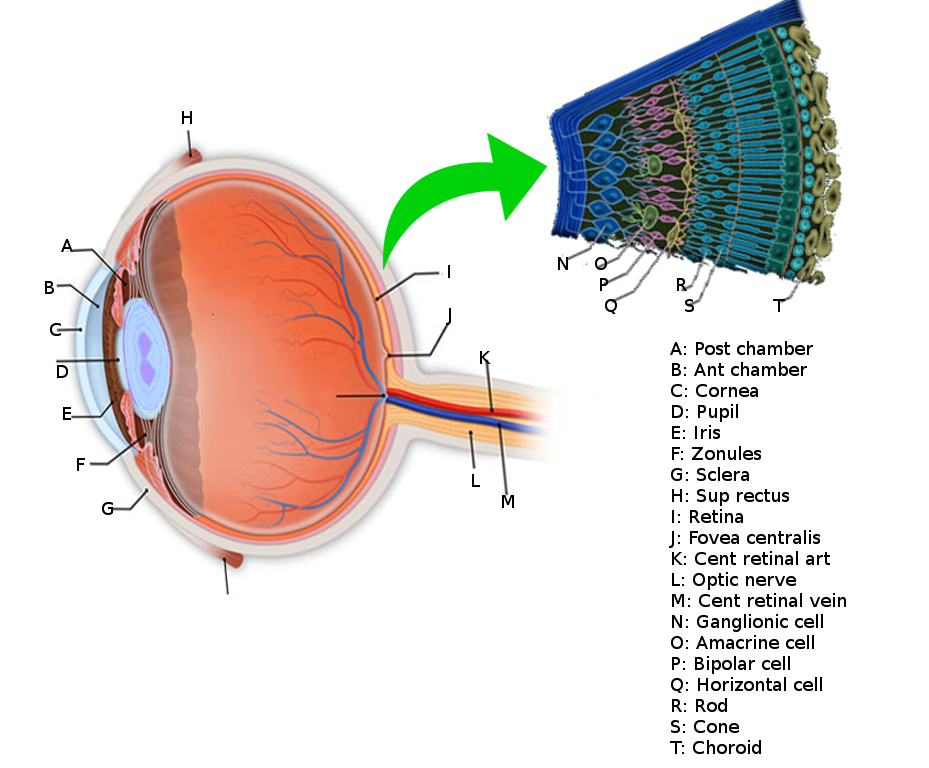
The retina is the innermost layer in the eye that is responsible for the visual processing that turns light energy from photons into three-dimensional images.[1] Located in the posterior portion of the eyeball, the retina is the only extension of the brain that can be viewed from the outside world and gives ophthalmologists a rare window into real-time pathology affecting the retina. Development of the retina is a long and complex process that begins during the fourth week of embryogenesis and continues into the first year of life. This long and complex embryonic development makes the retina vulnerable to genetic and environmental insults that can negatively affect retinal development. Retinal tissue develops to become the most metabolically expensive tissue in the human body, consuming oxygen more rapidly than any other tissue.[2] The retina is fed oxygen from a unique dual blood supply that divides the retina into outer and inner layers for more efficient oxygenation. The retina itself consists of six different cell lines divided into ten different layers, each playing a specific role in creating and transmitting vision. The different cell types perform a particular role and form functional circuits that specialize in detecting specific variations and movements of light.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The retina lines the entire posterior portion of the eye, except for the area of the optic nerve and extends anteriorly to end 360 degrees circumferentially at the ora Serrata, the junction between the retina and the ciliary body.
The retina is a layered structure with ten distinct layers of neurons interconnected by synapses. The cells subdivide into three basic cell types: photoreceptor cells, neuronal cells, and glial cells. The layers from the closest to the front anterior of the head towards the posterior of the head are as follows:
- Inner limiting membrane
- Nerve fiber layer (NFL)
- Ganglion cell layer
- Inner plexiform layer
- Inner nuclear layer
- Middle limiting membrane
- Outer plexiform layer
- Outer nuclear layer
- External limiting membrane
- The layer of rods and cones
Within these layers of the retina, we find multiple different types of cells with specific jobs that help transmit incoming photons into action potentials that the brain's cortices process into three-dimensional vision. The six different cell types in the retina include:
- Rods
- Cones
- Retinal Ganglion cells
- Bipolar cells
- Horizontal cells
- Amacrine cells
Rods
Rod cells can be traced back to approximately 500 million years ago when a piscine ancestor evolved rods to supplement the already pre-existing cones. It is presumed this evolutionary change allowed for organisms to have better survival in low-light settings. In humans, approximately 95% of the photoreceptors in our retina are rods, and they specialize in registering low-light levels, thus helping to create a black and white vision- known as scotopic vision. Rods are concentrated in the outer retina and their density increases as one moves outward towards the periphery of the retina, with there being zero rods in the central fovea. Rods differ from their color-sensing cone counterparts in a variety of ways. Rods have a slow speed of response, and their spatial acuity and contrast sensitivity are also very low, directly contrasting the rapid, high spatial acuity, and high contrast sensitivity of cones. Also, rods cannot function during the daytime as they are "photo-bleached" and need 20 minutes to recover, and only after 40 minutes after sunset or immersion into darkness can all the rods come online and help with creating scotopic vision.[3] Although the rods differ from the cones in ways that seem to make them inferior, the off-axis visual quality using rods is remarkably good. This phenomenon can be appreciated when objects are more visible when one looks just above or just below the intended object in a dark setting.[4] It is essential to understand that rods poor spatial acuity is not a result of any inherent inferiority in the rod cell, but a result of wiring. More rods converge onto a single retinal ganglion cell (RGC), whereas, cones keep a 1 to 1 ratio allowing for this difference in spatial acuity to emerge. Rods have a higher sensitivity to single photons of light than cones, whereas cones are more sensitive to specific wavelengths (colors) of light. The configuration of rods into the retinal system allows them to use their unique sensitivity to photons and integrate the photon signal for longer by converging multiple rods onto a single RGC and thus reducing background noise. Rod cells use glutamate as their neurotransmitter and synapse onto second-order bipolar cells at the outer plexiform layer.
Cones
The human retina contains approximately 6 to 7 million cones in total, comprising only 5% of the total number of retinal photoreceptors, but our visual acuity relies on as little as 100000 cones. Unlike rods, cones are less sensitive to photons in general but are better at responding to one of three specific (colors) wavelengths of light. Cone cells specialize in detecting either red light (64%), green light (32%), or blue light (2%) and concentrate primarily in the central area of the retina referred to as the macula- which also contains fovea. The central fovea contains neither rods nor even synapses, but only 100% cones which have an unobstructed view of the incoming light from the outside world. In the fovea, there is a 2 to 1 ratio of red and green specific cone cells, respectively, whereas cones in the peripheral surrounding macula are responsible for blue light detection. Cone cells help the brain process photopic vision, which involves color vision at varying light levels, which humans use in a majority of our day-to-day interactions. The high density of cones in the fovea enables the brain to differentiate between two points and in general cones allow for great spatial acuity even though rods are more sensitive to photons. Also, cones in the macula have a 1 to 1 ratio of synaptic connections to retinal ganglion cells, unlike rods which have a 3 to 1 ratio, allowing for each cone to contribute a greater amount of visual data to the overall picture the brain is trying to decipher. Cone cells differ from rod cells in a myriad of different ways. For example, cone cells can adapt to light rapidly and do not become saturated under constant light as rods do. After photobleaching cones can recover their membrane current within 20 milliseconds whereas rods can require upwards of 20 minutes to recover their membrane current. Cone cells release glutamate onto second-order bipolar cells located in the outer plexiform layer.[3]
Retinal Ganglion Cells
Retinal ganglion cells (RGC) are the retina's main output neuron, but also a third class of photoreceptors that are also photosensitive and help transmit both image-forming and non-image forming information that functions in the physiological processes of the circadian rhythm, modulation of melatonin release, and regulation of pupil size.[5] There are approximately 20 different RGCs, and 1 to 2% of all RGCs are intrinsically photosensitive, like their cone and rod counterparts, by their selective expression of the G-protein peptide neuromodulator called melanopsin.[6] RGCs receive both excitatory and inhibitory inputs from two types of intermediate neurons, amacrine cells, and bipolar cells. RGCs and amacrine cells form a functional subunit of on-off centers that allow for the brain to interpret a small dot moving at a distance.[7] RGC's send axonal projections that converge in the optic disc and pass through the lamina cribrosa unmyelinated, to not interfere with incoming light. RGC axons target the suprachiasmatic nucleus, olivary pretectal nucleus, intergeniculate leaflet, ventral division of the lateral geniculate nucleus, and preoptic area and thus assist with synchronization of circadian rhythms and the pupillary light reflex.[8][9]
Amacrine Cells
Amacrine cells are intermediate neurons that release the inhibitory neurotransmitter GABA or glycine. However, given their unique gap junction physiology, they can be both inhibitory or excitatory. There is great diversity among amacrine cells, and they fulfill a variety of jobs and functions within the retina; serving as the ultimate utility cell of the retina. The diverse lineage of retinal ganglion cells gives rise to an even more diverse diaspora of amacrine cells. Up to now, researchers have discovered 20 different ganglion cells with over 42 different amacrine cells accompanying them. This diversity among amacrine cells allows them to form dedicated functional microcircuits that allow the retina to detect different shades and movements of light in particular directions. These functional microcircuits are divided by the type of amacrine cells, which consists of a wide field, medium field, and narrow field amacrine cell. As their name suggests, there are different spectrums of information sharing depending on the particular field in which the amacrine cell works. For example, wide-field amacrine cells specialize in communicating horizontally across a single layer of the retina, assisting in horizontal information integration. Narrow-field amacrine cells are more narrow as their name suggests and penetrate more layers of the retina vertically, allowing for vertical integration of information. Stratification of amacrine cell output can be either pre-synaptic or post-synaptic and in conjunction with gap junctions, which allows for amacrine cells to be both inhibitory or excitatory despite releasing only inhibitory neurotransmitters. Amacrine cells from pre-synaptic inhibit bipolar cells terminals, and post-synaptically inhibit RGC dendrites. Recently, scientists discovered that amacrine cells also have a paracrine function, with some varieties releasing dopamine.[7][9]
Bipolar Cells
Bipolar cells are second-order long-projection neurons, named after their axons 180-degree orientation, that receive visual inputs from photoreceptors (rods and cones) and projects their axons onto retinal ganglion cells. Thirteen different types of bipolar cells divide into rod bipolar cells and cone bipolar cells, depending on the cell from which cell they receive inputs. Bipolar cells form circuits with other photoreceptors that provide the basic elementary blocks of vision such as chromatic composition, polarity, contrast, and temporal profile of incoming visual stimuli. Each cone bipolar cell and rod bipolar cell is further subdivided depending on whether it depolarizes in response to light (ON-bipolar cells) or those that hyperpolarize (OFF-bipolar cells). Cone bipolar cells are either ON or OFF type, whereas rod bipolar cells are only the ON type. Rods specialize in scotopic vision and thus only need to determine whether or not photons are striking the retina quantitatively, thus ON bipolar cells are adequate for this binary function. Cones cells provide information for the photopic vision that can differentiate fine details, movements, and colors, and this require both ON and OFF bipolar cells to qualitatively differentiate incoming photons. Bipolar cells link the inner and outer layers of the retina by forming a synaptic connection with rods and cones in the inner plexiform layer (IPL) of the retina. The IPL functions as a switchboard whereby different bipolar cells stratify at five different layers within the IPL, carrying different forms of elementary visual information to specific groups of RGCs and amacrine cells. Bipolar cells form distinct relationships with RGCs, amacrine, and horizontal cells. Amacrine cells pre-synaptically inhibit bipolar cell terminals in the IPL. Horizontal cells give GABAergic inhibitory inputs into bipolar cells. Therefore, bipolar cells receive glutamatergic inputs from rods and cones, and GABAergic inputs from horizontal cells, and in turn, bipolar cells provide glutamatergic excitatory input to RGCs and amacrine cells. This form of parallel information processing allows highly pre-processed excitatory inputs to become the elementary building blocks of vision.[10][9]
Horizontal Cells
Horizontal cells are involved in modulating information transfer between bipolar cells and photoreceptors and are involved with helping eyes adjust to both bright light and low light conditions. They have wide and diffuse horizontal projections and couple to their neighbors via gap junctions. There are three distinct types of horizontal cells in the retina with their cell bodies concentrated towards the outer retina located mostly in the inner nuclear layer. Horizontal cells are GABAergic interneurons that provide inhibitory inputs to bipolar cells as well as inhibitory feedback to both rods and cones. However, this is a point of great contention among scholars. Postulations are that horizontal cells do not use GABA for inhibition, but instead, they inhibit bipolar cells and photoreceptors by modulating the pH within the synaptic cleft. Horizontal cells form contacts in the outer plexiform layer that convey polarity, spectral sensitivity, speed, and structure the spatial receptive field. Horizontal cells amplify signals from ON-OFF centers by providing lateral inhibitory GABAergic inputs to the surrounding bipolar cells encircling the ON-center or OFF-center bipolar cells. By antagonizing the surrounding bipolar cells, horizontal cells help support contrast enhancement via binary signaling and providing two-point differentiation. Horizontal cells play a key role in sheathing bipolar cells, invaginating contacts with dendrites of ON cone bipolar cells and have basal contacts with OFF cone bipolar cells.[11][9][12]
Central vs. Peripheral Retina
The central retina differs from the peripheral retina in both thickness and composition. The central retina is thicker and is packed densely with cones, while the peripheral retina is thinner and is composed of mostly rods. The outer nuclear layer is the layer that contains the rods and cones and is uniform in thickness but differs in composition of rods and cones, with more cones located centrally and with more rods in the periphery but this layer, unlike the retina as a whole, is uniform in thickness. One can appreciate the variability in the thickness of the retina in the inner nuclear layer. The inner nuclear layer contains a greater density of synaptic connections between cones and bipolar cells, as well as higher concentrations of horizontal cells and amacrine cells. Also, cones have a 1 to 1 synaptic convergence ratio with second-order neurons given that they are used for high acuity visual processing whereas rods from the periphery have a ratio closer to 3 to 1 leading to differences in the thickness.[13]
Muller Glial Cells
Radial glial cells of the retina, also known as Muller cells, are in the outer limiting membrane (OLM) of the retina and form adherens junctions between Muller cells and rods and cones in the inner segments. The retina's inner limiting membrane (ILM) is composed of laterally contacting Muller cell synaptic boutons and other basement membrane parts.
Inner limiting membrane
The ILM is the retina's inner surface bordering the vitreous humor and thereby forming a diffusion barrier between the neural retina and vitreous humor. The ILM contains laterally contacting Muller cell synaptic boutons and other basement membrane parts.
Nerve fiber layer (NFL)
The nerve fiber layer is the second innermost layer of the retina from the vitreous. Patients with retinitis pigmentosa may have a measurable degree of RNFL thinning as determined by OCT.[14]
Ganglion cell layer
This layer contains the retinal ganglion cells (RGCs) and displaced amacrine cells. As a rule of thumb, smaller RGCs dendrites arborize in the inner plexiform layer while larger RGCs dendrites arborize in other layers.
Inner plexiform layer
The inner plexiform layer is an area comprised of a dense reticulum of fibrils formed by interlaced dendrites of RGCs and cells of the inner nuclear layer.
Inner nuclear layer
This layer of the retina contains the cell bodies of bipolar cells, horizontal cells, and amacrine cells.
Outer plexiform layer
This layer of the retina contains a neuronal synapse of between rods and cones with the footplate of horizontal cells. Capillaries are also found to be primarily running through the outer plexiform layer.
Outer nuclear layer
This layer contains the rod and cone granules that sense photon, extensions from the rod, and cone cell bodies.
External limiting membrane
This layer contains the bases of the rod and cone photoreceptors cell bodies. The ELM forms a barrier between the subretinal space, into which the inner and outer segments of rods and cones project to be in close association with the pigment epithelial layer behind the retina, and the neural retina proper.
Retinal pigment epithelium
The retina is supported by the retinal pigment epithelium (RPE), which has many functions including vitamin A metabolism, maintenance of the blood-retina barrier, phagocytosis of photoreceptor outer segments, production of mucopolysaccharide matrix surrounding the outer segments of the retina, and active transport of materials into and out of the RPE.
Embryology
The retina is derived from ectodermal and neural crest embryonic cells and starts development as two optic vesicles on the lateral sides of the embryonic forebrain that invaginate and form two optic grooves. The optic grooves continue to develop and by the eighth week of development the retina has formed into the precursor layers from outermost to innermost: the pigmented layer, outer limiting membrane, proliferation zone, external neuroblastic layer, transient fiber layer, internal neuroblastic layer, nerve fiber layer, and last but not least the internal limiting membrane. The internal neuroblastic layer will develop into the light-sensing rods and cones. The outer pigmented layer consists of cells that directly originate from the optic neuroepithelium. Neural crest cells migrate towards the eye and develop into choroidal melanocytes, which provide the pigment that will prevent the scatter of light once the eye is fully developed. These layers of the retina continue to develop in darkness until the child is born.
Blood Supply and Lymphatics
The retina demonstrates the highest rate of oxygen consumption of any tissue in the human body and, therefore, requires a steady and large volume of oxygenated hemoglobin to nourish it. The retina possesses a dual blood supply to accommodate this high demand; it receives supply by the choroid and the branches of the ophthalmic artery. Blood leaves the heart from the aorta and enters the common carotid artery, which divides into the internal and external carotid vessels. From this junction, the internal carotid enters the skull and just distal to the cavernous sinus, the first tributary off of the internal carotid is the ophthalmic artery. The ophthalmic artery gives rise to both the central retinal artery and the posterior ciliary arteries which supply the retina from different angles. The central retinal artery is the first branch of the ophthalmic artery and runs inside the dura just beneath the optic nerve, and travels with the optic nerve through the optic disc and supplies the cells in the macula. The posterior ciliary artery divides into the short and long posterior ciliary arteries that penetrate through the sclera and provide blood flow to the posterior uveal tract. Blood flow to the retina remains constant regardless of intraocular pressure, systemic blood pressure, and is independent of sympathetic autoregulation. Instead, retinal blood flowregulation is by local factors such as nitric oxide, prostaglandins, endothelin, but most importantly, arterial carbon dioxide tension. Much akin to the brain, retinal blood flow will increase in response to increased carbon dioxide and decrease in response to lower levels of carbon dioxide. The two branches are the posterior ciliary arteries which supply the outer and middle retina and the central retinal arteries which supply the inner retina. The choroid is the posterior part of the uveal tract that nourishes the outer layers of the retina. The choroid itself gets blood from the long and short posterior ciliary arteries. Capillaries are found within all parts of the retina from the innermost nerve fiber layer to the outermost outer plexiform layer and occasionally in the outer nuclear layer. Nutrients from the vasculature of the choriocapillaris behind the pigment epithelium layer supply the delicate photoreceptor layer. The photoreceptors and the larger portion of the outer plexiform layer obtain nourishment from the choriocapillaris indirectly, as opposed to the inner retinal layers which receive supply from the superficial and deep capillary plexuses formed by branches of the central artery of the retina. Inner layers of the retina are known to show the highest sensitivity to hypoxic challenges, whereas the outer retina exhibits greater resistance to hypoxic stress.[2]
Nerves
The retina is an extension of the optic nerve, also known as cranial nerve II. After light reaches the photoreceptor cells, rods and cones, action potentials are conveyed to the brain through the optic nerve. The optic nerve project through the optic stalks back into the optic chiasm, to the lateral geniculate nucleus, and to the visual cortex in the posterior occiput. The optic nerve is relatively close to the fovea, but at that point, there are no cones- this results in a blind spot.
Clinical Significance
In a retinal detachment, layers of rods and cones become detached from the RPE. The separation of the neurosensory layer of the retina from the outermost pigmented epithelium leads to the degeneration of photoreceptors and subsequent vision loss. Symptoms of early retinal detachment include flashes and floaters in the affected eye or a veil/curtain type of vision loss that is constant (vs. transient loss due to amaurosis fugax). Treatment includes lasering around the detached area to re-adhere the retina to the underlying RPE or doing a vitrectomy and filling the eye with oil to press the retina back onto the RPE. Common causes of retinal detachment include trauma, hypertension, and diabetic retinopathy.
Media
References
Masland RH. The neuronal organization of the retina. Neuron. 2012 Oct 18:76(2):266-80. doi: 10.1016/j.neuron.2012.10.002. Epub 2012 Oct 17 [PubMed PMID: 23083731]
Level 3 (low-level) evidenceKaur C, Foulds WS, Ling EA. Hypoxia-ischemia and retinal ganglion cell damage. Clinical ophthalmology (Auckland, N.Z.). 2008 Dec:2(4):879-89 [PubMed PMID: 19668442]
Lamb TD. Why rods and cones? Eye (London, England). 2016 Feb:30(2):179-85. doi: 10.1038/eye.2015.236. Epub 2015 Nov 13 [PubMed PMID: 26563661]
Anderson SJ, Mullen KT, Hess RF. Human peripheral spatial resolution for achromatic and chromatic stimuli: limits imposed by optical and retinal factors. The Journal of physiology. 1991 Oct:442():47-64 [PubMed PMID: 1798037]
Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Archiv : European journal of physiology. 2007 Aug:454(5):849-55 [PubMed PMID: 17351786]
Level 3 (low-level) evidenceHannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002 Jan 1:22(1):RC191 [PubMed PMID: 11756521]
Level 3 (low-level) evidenceMasland RH. The tasks of amacrine cells. Visual neuroscience. 2012 Jan:29(1):3-9 [PubMed PMID: 22416289]
Level 3 (low-level) evidenceHattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. The Journal of comparative neurology. 2006 Jul 20:497(3):326-49 [PubMed PMID: 16736474]
Level 3 (low-level) evidenceEuler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nature reviews. Neuroscience. 2014 Aug:15(8):507-19 [PubMed PMID: 25158357]
Level 3 (low-level) evidenceHoon M, Okawa H, Della Santina L, Wong RO. Functional architecture of the retina: development and disease. Progress in retinal and eye research. 2014 Sep:42():44-84. doi: 10.1016/j.preteyeres.2014.06.003. Epub 2014 Jun 28 [PubMed PMID: 24984227]
Level 3 (low-level) evidenceSchubert T, Huckfeldt RM, Parker E, Campbell JE, Wong RO. Assembly of the outer retina in the absence of GABA synthesis in horizontal cells. Neural development. 2010 Jun 18:5():15. doi: 10.1186/1749-8104-5-15. Epub 2010 Jun 18 [PubMed PMID: 20565821]
Level 3 (low-level) evidenceDeniz S, Wersinger E, Schwab Y, Mura C, Erdelyi F, Szabó G, Rendon A, Sahel JA, Picaud S, Roux MJ. Mammalian retinal horizontal cells are unconventional GABAergic neurons. Journal of neurochemistry. 2011 Feb:116(3):350-62. doi: 10.1111/j.1471-4159.2010.07114.x. Epub 2010 Dec 13 [PubMed PMID: 21091475]
Level 3 (low-level) evidenceVenters SJ, Mikawa T, Hyer J. Central and peripheral retina arise through distinct developmental paths. PloS one. 2013:8(4):e61422. doi: 10.1371/journal.pone.0061422. Epub 2013 Apr 16 [PubMed PMID: 23613848]
Level 3 (low-level) evidenceWalia S, Fishman GA, Edward DP, Lindeman M. Retinal nerve fiber layer defects in RP patients. Investigative ophthalmology & visual science. 2007 Oct:48(10):4748-52 [PubMed PMID: 17898300]