Introduction
Meningitis is an infection/inflammation of the brain and spinal cord surrounding membranes known as the meninges. Meningococcal meningitis is the term used to describe a bacterial form of meningitis caused by Neisseria meningitidis. This form of meningitis is associated with high morbidity and mortality. Meningococcal meningitis is a medical emergency for which symptoms can range from transient fever to fulminant bacteremia and septic shock.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Neisseria meningitides is the organism responsible for meningococcal meningitis and is the second most common causative organism for bacterial meningitis in the United States.[1] This bacterium is an anaerobic, bean, or kidney-shaped gram-negative diplococci. It grows in chocolate agar and blood agar at room temperature with 5%-10% atmospheric carbon dioxide. Neisseria meningitides are catalase-positive, oxidize glucose and maltose that changes pH, and helps to differentiate from gonococci, which cannot oxidize maltose. The organism grows in selective Thayer-Martin media, which contains antibiotics like vancomycin, colistin, and nystatin by preventing the growth of other gram-negative bacteria, gram-positive bacteria, and yeast. N. meningitidis colonizes mostly in the naso-and oropharynx but can colonize in other body parts like the anal mucosa, conjunctiva, and urogenital tracts. It possesses multiple virulent factors: Pilli, opacity proteins, lipo-oligosaccharides, capsular polysaccharide, and factor H binding protein. The polysaccharide capsule protects the bacterium from complement-mediated phagocytosis and lysis.[2]
Serotypes are classified according to the polysaccharide capsule it contains. Out of 13 serotypes, A, B, C, X, Y, Z, W-135, and L are mostly responsible for human disease.[3] Unencapsulated strands found in asymptomatic carriers rarely have been the cause of invasive disease.[4] Lipo-oligosaccharide is similar to lipopolysaccharide of gram-negative bacilli.[5] LOS acts as endotoxin and activates the pro-inflammatory cytokine pathway of the host to cause meningococcal sepsis.[6] LOS interacts with immune cells of the host to initiate the release of inflammatory mediators like TNF- alpha, IL-1, IL-6, and INF-gamma to cause shock.[7][8][9]
Risk Factors
Neisseria meningitidis infection depends upon various environmental and host factors. Based on experience with military recruits, the nasopharyngeal carrier state is the primary factor for the transmission and development of meningitis.[10] That is why tetravalent vaccination against N. meningitides has been started since 1972 among military recruits in the USA.[11] Early and late complement deficiency has been associated with increased susceptibility to meningococcal infection.[12] There is an increased risk of acquiring an invasive meningococcal infection in patients with HIV/AIDS.[13] The incidence of meningococcal infection is more among people who have sex with others with HIV than those who have sex with people without HIV.[14] People under eculizumab have about 1000 or 2000 times increased risk of fatal meningococcal infection.[15]
Epidemiology
N. meningitidis was estimated to be responsible for 1.2 million cases of infection per year, as well as approximately 135,000 deaths worldwide.[16] Infants and adolescents are most vulnerable to developing meningococcal disease due to elevated rates of nasopharyngeal colonization and waning maternal antibodies. In the United States, the annual incidence rate of meningococcal meningitis between 2006 and 2015 was 0.28 cases per 100000, and 2.50 cases per 100000 for infants aged less than one year.[17] Due to large and persistent epidemics, the highest incidence rates of meningococcal disease are found in the “meningitis belt” of sub-Saharan Africa, with rates ranging from 20 to 1000 per 100000.[18]
Furthermore, the serogroups causing meningococcal disease vary geographically. Meningococcus serogroup A predominates in Africa and regions of Asia, whereas serogroups B, C, and Y predominate in other areas, including North America and Europe.[19] In the United States, serogroups B, C, and Y are the predominant serogroups causing meningococcal meningitis, each accounting for one-third of cases.[20] Among those, serogroup B is the cause of 50% of meningococcal cases in infants, whereas serogroup C is commonly seen in adolescents, and serogroups B and Y in older adults.[16]
Between 2006 and 2015:[17]
- Annual incidence changed from 0.40 cases to 0.14 cases per 100,000.
- For serogroup B infection, incidence changed from 0.25 to 0.05 cases per 100,000.
- For serogroup C infection, incidence changed from 0.38 to 0.01 cases per 100,000.[20]
- For serogroup Y infection, incidence changed from 0.42 to .02 cases per 100,000.
- Besides those serogroups, the incidence of infection from other serogroups remained constant.
The incidence of infection in the male population is slightly higher than females. Among races, the incidence rate in African-Americans is found to be 0.27 cases per 100,000, whereas the incidence rate of infection in other races is 0.20 cases per 100,000. Despite medical advancements in the prevention of this disease, mortality rates remain in a range between 5% and 15% for those treated, and as high as 50% in those without treatment.[21]
Pathophysiology
The pathophysiology of N. meningitidis involves a series of sequential steps. The process begins when the bacterium colonizes the nasopharynx. The only known reservoir for N. meningitidis is the upper respiratory tract, although only a few will develop invasive disease.[22] The bacterium incubates for a period to 1 to 10 days and then further penetrates the submucosa. In 10 to 20% of cases, the bacterium invades the bloodstream. Once present in the plasma, host defenses, which include bactericidal antibodies, complement, and phagocytic cells, may prevail and eliminate bacteria. In cases, where host defenses fail to clear bacteria, the patient enters the bacteremic phase. Bacteria may now invade meninges and other local sites, which can rapidly lead to meningitis and fatal septicemia.[23]
History and Physical
Prompt recognition and immediate initiation of treatment are of utmost importance in the management of bacterial meningitis. The meningococcal disease presents with signs and symptoms which can vary from an undifferentiated febrile illness to fulminant septic shock. Rapid progression of symptoms over hours is typical and can be helpful when trying to differentiate meningitis from a self-limiting viral infection.[10]
The classic triad of neck stiffness, fever, and altered mental status is a more specific sign for meningitis. Infants can present with a variety of non-specific symptoms, which include lethargy, irritability, and in some cases bulging fontanelles. Older children and adults will present with headaches, fever, photophobia, vomiting, neck stiffness, and altered mental status. Meningococcal disease often presents with a petechial rash to the lower extremities, which occurs following the initial non-specific symptoms, although this symptom can also be present in pneumococcal meningitis.[24] Out of fever, neck stiffness, altered mental status, and rash, 89% of the patients had at least two signs.[25] The patients can also have myalgias, which are more painful than those associated with viral influenza. The provider should also be concerned about the signs and symptoms of early sepsis, like leg pain, cold hands & feet, and abnormal skin color (e.g., pallor or mottling).[26]
Physical Examination
Patients can present with abnormal vital signs, including fever, tachypnea, tachycardia, and hypotension. Hypotension with elevated pulse rate is suggestive of early vascular instability. The patient should be fully undressed to look for petechiae and ecchymosis and to perform a thorough skin exam. Meningeal irritability can be confirmed by provocative tests like Kernig and Brudzinki sign. When the knee of the patient in the supine position with flexed hip and knee at the right angle is slowly extended, there is the appearance of resistance or pain on an extension beyond 135 degrees i.e., positive Kernig sign.[27]
In the same way, Brudzinski sign is positive when the patient is in the supine position and shows reflex flexion of the hips and knee after passive flexion of the neck. At the same time, the examiner keeps one hand behind the patient’s head and other on the chest, preventing the rising of the patient from a supine position.[27] The absence of these signs does not exclude systemic meningococcal infection. A thorough neurologic exam should be performed looking for alteration in mental status, as well as any focal deficits.
Evaluation
Initial blood tests should include full blood cell count, electrolytes, coagulation studies, and blood cultures.[28] Lumbar puncture is necessary for a definitive diagnosis, but if clinical suspicion is high, diagnostic tests should not delay antibiotic administration.
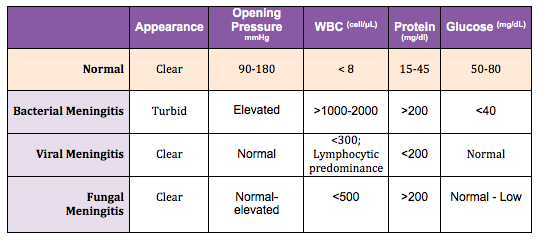
Lumbar Puncture: Diagnosis can be made from bacterial isolation of N. meningitidis from cerebrospinal fluid, blood, and skin lesions. CSF can be obtained via lumbar puncture. CSF fluid typically reveals high opening pressure, pleocytosis (WBC more than 1000/microliters), high protein concentration (more than 500 mg/dl), low glucose (less than 45 mg/dl or less than 2.5 mmol/L), and CSF: serum glucose ratio of less than 0.4.[19] Of note, CSF sterilization may occur within the first two hours of antibiotic administration, but again this should not delay antibiotic treatment.[29]
CSF fluid should also be sent for Gram staining, standard culture, and polymerase chain reaction (PCR). Gram staining is diagnostic in 85% of patients with meningococcal meningitis. Bacteriologic culture establishes a definitive diagnosis. These should be grown on chocolate blood agar at 35 degrees C. N. meningitidis tends to be positive in around 50% of blood cultures and approximately 75% of cerebrospinal fluid. The cerebrospinal fluid assay can be done with latex agglutination, PCR, rapid dipstick test, and loop-mediated isothermal amplification (LAMP). CSF studies with PCR can also be obtained, although these commonly tend to yield false-negative results. PCR has more advantages than culture for diagnosis of meningococcal infection as it is rapid, can detect different strains of even non-viable bacteria.[30] LAMP is a highly accurate rapid test for the diagnosis of meningococcal infection at an emergency than PCR or culture.[31][32]
Imaging: Imaging studies, specifically CT scans, can also be performed if the patient meets the criteria for CT before LP. The criteria include age greater than 60, presence of focal neurologic deficits, altered mental status, immunodeficiency, new-onset seizures, history of central nervous system disease, and papilledema.[23]
Treatment / Management
Meningococcal meningitis is a medical emergency presenting with severe sepsis syndrome, fever, petechiae, and ecchymosis require prompt resuscitation and antibiotic administration.[33]
Resuscitation: Meningococcal disease can cause life-threatening organ dysfunction. On arrival, the patient’s hemodynamic stability and need for acute interventions should be evaluated. Early intubation should be considered in patients with evidence of airway compromise, ongoing shock, intractable seizures, or elevated intracranial pressure.[28] Given meningococcal disease often results in vascular collapse and septic shock, resuscitation should also include stabilizing blood pressure and providing sufficient tissue perfusion. Hemodynamic support should consist of fluid resuscitation and the addition of vasopressors as needed.
Antibiotics: Antibiotic dose should be given as soon as meningitis is suspected and should not be delayed awaiting confirmatory studies. Lumbar puncture is performed as soon as possible as parenteral antibiotic therapy clears out meningococci from CSF in less than six hours.[34] Local guidelines should dictate antibiotic treatment. On initial encounter when the patient presents with an undifferentiated acute bacterial meningitis, administration of broad-spectrum antibiotics is appropriate pending bacterial isolation. Seven days course of antibiotic therapy is usually sufficient to treat suspected cases of meningococcal meningitides.[35] Initial treatment for adults should include the administration of a third-generation cephalosporin (such as cefotaxime or ceftriaxone, i.e., twice daily dose) in combination with vancomycin, with the addition of ampicillin for those greater than 50-years-old to cover L. monocytogenes.[35] (A1)
Third-generation cephalosporins have great coverage against N. meningitides.[36] Once N. meningitis has been confirmed, penicillin (IV/IM usual dose 300,000 units/kg per day with an upper limit of 24 million units/day or 4 million units IV every 4 hours) is recommended as monotherapy due to its low cost and narrow spectrum.[24] For minimum inhibitory concentration (MIC) <0.1 mcg/ml, the patient treated with penicillin[35] whereas MIC of 0.1 to 1 mcg/dl, third-generation cephalosporin is preferred. In case patients who cannot tolerate beta-lactam or during epidemics or resource-limited settings, chloramphenicol (100 mg/kg per day IV up to maximum dose 4 g/kg) is the choice of treatment for meningococcal meningitides.[37](A1)
Steroids: Traditionally, patients receive an empiric dose of steroids (dexamethasone), but the use of corticosteroids is controversial and may be of more benefit in patients with meningitis due to S.pneumoniae and H.influenzae.[23]
Prevention
The N. meningitidis can be prevented by applying measures under the following subcategories.[38]
Pre-exposure:
Droplet Precaution: The patient with suspected or confirmed N. meningitidis should follow droplet precaution. This should be continued until after 24 hours of effective antibiotics administration.
Vaccination: In the United States, a quadrivalent meningococcal conjugate vaccine (Menactra or Menveol) is administered to:[20]
1. All individuals between age 11 and 18 years of age i.e., at age 11 or 12, and a booster dose at age 16 years.
2. Individuals 10 years or less or 19 years or less of age with increased risk of invasive meningococcal disease:
- Travelers to hyperendemic or epidemic area of meningococcal disease
- Military recruits
- Lab worker handling N. meningitidis
- Functional or anatomic asplenia
- Complement component deficiency
- Individual under treatment with C5 inhibitors (eculizumab/ravulizumab)
Meningococcal vaccines (both ACYW and serogroup B) are given two weeks before administering the first case of the C5 inhibitor.
Avoidance of risk factors.
Post-Exposure:
Antimicrobial Prophylaxis:
Close contact is defined as individuals with more than eight hours of contact in less than three feet of proximity to patient or person directly exposed to patient’s secretions during seven days before the onset of symptoms and until 24 hours after initiation of antibiotic therapy.[20] Close contacts and healthcare workers who had contact with patient’s secretions should receive chemoprophylaxis with either ciprofloxacin, rifampin, or ceftriaxone.[39]
In rare cases of ciprofloxacin-resistant N. meningitidis, rifampin, and ceftriaxone are used as alternatives for prophylaxis. Patients under C5 inhibitors (eculizumab and ravulizumab) should receive antibiotic prophylaxis of penicillin or macrolide (penicillin allergy) in addition to vaccination for the entire duration of therapy. Close contacts receiving prophylaxis should be under surveillance for ten days following exposure. Surveillance and counseling about the sign-symptom of meningococcal infection would ensure prompt treatment of any secondary case.
Chemoprophylaxis should be given to patients with invasive meningococcal disease with nasopharyngeal carriage of N.meningitidis before discharge from the hospital to prevent transmission to close contacts.[40]
Differential Diagnosis
Differential diagnoses should include a myriad of neurologic diseases such as
- Encephalitis (bacterial/viral)
- Brain abscess
- Malignancy
- Intracranial bleed
- Cerebrovascular accident[41]
Other relevant differentials are
- Tetanus
- Rabies
- Medications causing serotonin syndrome/neuroleptic malignant syndrome
- Sinusitis
Meningococcal meningitidis progresses to meningococcemia with a purpuric rash which is difficult to differentiate from
- Sepsis
- Disseminated gonococcemia
- Rocky Mountain Spotted Fever
- Typhus(endemic or epidemic)
- Leukocytoclastic vasculitis
- Hemorrhagic dengue
- Ehrlichiosis
- Anaplasmosis
- Borreliosis(Lyme disease)
- Thrombotic thrombocytopenic purpura
- Idiopathic thrombocytopenic purpura
Prognosis
According to the CDC, current mortality rates of the patient with meningococcal meningitis in the United States are approximately 10% to 15 % with treatment [20] and up to 50% if left untreated. Prompt antibiotic administration, especially within one hour, has been proven to improve morbidity and mortality, as well as prevent complications such as increased intracranial pressure and septic shock.[42][43]
The factors like shock, presence of focal deficits, mental obtundation or coma, purpuric or ecchymotic rash, absence of meningeal signs, low or normal blood leukocyte count, age of more than 60 years, presence of anemia, thrombocytopenia, low erythrocyte sedimentation rate (or C-reactive protein level), low blood concentrations of antithrombin or proteins S and C, high blood levels of plasminogen activator inhibitor 1 (PAI-1), malignancy, intracranial bleed, and cerebrovascular accident, are considered as a poor prognostic factor for meningococcal meningitides patients.[44] Female gender and cases in the outbreak have also been associated with high case- fatality rates among meningococcal disease patients.[45][46]
Complications
Complications of meningococcal meningitis can arise early or late in the disease course and can adversely impact morbidity and mortality. Intracranial complications include cerebral edema, vascular alterations, and hydrocephalus. Extracranial complications include septic shock, disseminated intravascular coagulation, multiorgan failure, including acute adrenal crisis due to adrenal hemorrhage (Waterhouse- Friderichsen syndrome), and electrolyte disturbances.[47]
Late complications of meningococcal meningitis include chronic pain, skin scarring, and neurologic impairment. Other common complications include hearing impairment, visual impairment, and seizures. Hearing tests are recommended four weeks after hospital discharge. Post-traumatic stress disorder can also ensue; management may require psychiatric or psychological intervention.[42]
As with any critical illness, other complications could include prolonged ventilatory weaning, tracheostomy care, feeding tube care, prolonged physical and occupation rehabilitation, development of polyneuropathy, and polymyopathy of critical illness, as well as the development of secondary infections and wounds.
Deterrence and Patient Education
Meningococcal disease is best prevented by vaccination. The implementation of vaccination programs has led to reductions in the incidence of meningococcal disease. Current US guidelines recommend vaccination with the quadrivalent vaccine at ages 11 to 12 and revaccination at age 16, which will ensure continued protection for an additional 3 to 5 years when they are deemed to be at higher risk. Given the ability of meningococcus to cause rapidly fatal disease, prevention strategies are of utmost importance.[48]
Enhancing Healthcare Team Outcomes
Meningococcal meningitis is a possibly fatal disease if not promptly recognized and treated. A multidisciplinary approach should be utilized to improve patient outcomes and deliver comprehensive care.[49] The team should include emergency providers, nursing staff, laboratory technicians, pharmacists, intensivists, and infectious disease specialists.
Coordination of these different professionals delivering care as a team will ultimately lead to prompt diagnosis and timely antibiotic administration, which in turn will significantly decrease morbidity and mortality in these patients. Physical and occupational rehabilitation is needed to minimize long-term complications and improve the quality of life in patients with meningococcal meningitis.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A, Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998-2007. The New England journal of medicine. 2011 May 26:364(21):2016-25. doi: 10.1056/NEJMoa1005384. Epub [PubMed PMID: 21612470]
Feldman C, Anderson R. Meningococcal pneumonia: a review. Pneumonia (Nathan Qld.). 2019:11():3. doi: 10.1186/s41479-019-0062-0. Epub 2019 Aug 25 [PubMed PMID: 31463180]
Nguyen N, Ashong D. Neisseria Meningitidis. StatPearls. 2024 Jan:(): [PubMed PMID: 31751039]
Harrison LH. Epidemiological profile of meningococcal disease in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Mar 1:50 Suppl 2(S2):S37-44. doi: 10.1086/648963. Epub [PubMed PMID: 20144015]
Level 2 (mid-level) evidenceKahler CM, Stephens DS. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Critical reviews in microbiology. 1998:24(4):281-334 [PubMed PMID: 9887366]
Persa OD, Jazmati N, Robinson N, Wolke M, Kremer K, Schweer K, Plum G, Schlaak M. A pregnant woman with chronic meningococcaemia from Neisseria meningitidis with lpxL1-mutations. Lancet (London, England). 2014 Nov 22:384(9957):1900. doi: 10.1016/S0140-6736(14)61645-7. Epub 2014 Nov 21 [PubMed PMID: 25457917]
Level 3 (low-level) evidenceBechtle F. [Dental activity in Basutho-Qwa-Qwa]. Zahnarztliche Praxis. 1977 Jun 17:28(12):293-5 [PubMed PMID: 278334]
Girardin E, Grau GE, Dayer JM, Roux-Lombard P, Lambert PH. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. The New England journal of medicine. 1988 Aug 18:319(7):397-400 [PubMed PMID: 3135497]
Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun JN, Halvorsen S, Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. The Journal of infectious diseases. 1989 Feb:159(2):195-204 [PubMed PMID: 2492587]
Broome CV. The carrier state: Neisseria meningitidis. The Journal of antimicrobial chemotherapy. 1986 Jul:18 Suppl A():25-34 [PubMed PMID: 3091563]
Artenstein MS, Gold R, Zimmerly JG, Wyle FA, Schneider H, Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. The New England journal of medicine. 1970 Feb 19:282(8):417-20 [PubMed PMID: 4983754]
Fijen CA, Kuijper EJ, te Bulte MT, Daha MR, Dankert J. Assessment of complement deficiency in patients with meningococcal disease in The Netherlands. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999 Jan:28(1):98-105 [PubMed PMID: 10028078]
Miller L, Arakaki L, Ramautar A, Bodach S, Braunstein SL, Kennedy J, Steiner-Sichel L, Ngai S, Shepard C, Weiss D. Elevated risk for invasive meningococcal disease among persons with HIV. Annals of internal medicine. 2014 Jan 7:160(1):30-7. doi: 10.7326/0003-4819-160-1-201401070-00731. Epub [PubMed PMID: 24166695]
Level 2 (mid-level) evidenceBozio CH, Blain A, MacNeil J, Retchless A, Weil LM, Wang X, Jenkins LT, Rodriguez-Rivera LD, Jarashow C, Ngo V, Hariri S, Mbaeyi SA, Oliver S. Meningococcal Disease Surveillance in Men Who Have Sex with Men - United States, 2015-2016. MMWR. Morbidity and mortality weekly report. 2018 Sep 28:67(38):1060-1063. doi: 10.15585/mmwr.mm6738a4. Epub 2018 Sep 28 [PubMed PMID: 30260947]
Applegate AO, Fong VC, Tardivel K, Lippold SA, Zarate S. Notes from the Field: Meningococcal Disease in an International Traveler on Eculizumab Therapy - United States, 2015. MMWR. Morbidity and mortality weekly report. 2016 Jul 15:65(27):696-7. doi: 10.15585/mmwr.mm6527a3. Epub 2016 Jul 15 [PubMed PMID: 27414068]
Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods in molecular biology (Clifton, N.J.). 2012:799():1-20. doi: 10.1007/978-1-61779-346-2_1. Epub [PubMed PMID: 21993636]
Level 3 (low-level) evidenceMacNeil JR, Blain AE, Wang X, Cohn AC. Current Epidemiology and Trends in Meningococcal Disease-United States, 1996-2015. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Apr 3:66(8):1276-1281. doi: 10.1093/cid/cix993. Epub [PubMed PMID: 29126310]
Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012 May 30:30 Suppl 2():B26-36. doi: 10.1016/j.vaccine.2011.12.032. Epub 2011 Dec 15 [PubMed PMID: 22178525]
Bosis S, Mayer A, Esposito S. Meningococcal disease in childhood: epidemiology, clinical features and prevention. Journal of preventive medicine and hygiene. 2015 Aug 31:56(3):E121-4 [PubMed PMID: 26788732]
Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2013 Mar 22:62(RR-2):1-28 [PubMed PMID: 23515099]
Fitzgerald D, Waterer GW. Invasive Pneumococcal and Meningococcal Disease. Infectious disease clinics of North America. 2019 Dec:33(4):1125-1141. doi: 10.1016/j.idc.2019.08.007. Epub [PubMed PMID: 31668194]
Hill DJ, Griffiths NJ, Borodina E, Virji M. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clinical science (London, England : 1979). 2010 Feb 9:118(9):547-64. doi: 10.1042/CS20090513. Epub 2010 Feb 9 [PubMed PMID: 20132098]
Johri S, Gorthi SP, Anand AC. Meningococcal Meningitis. Medical journal, Armed Forces India. 2005 Oct:61(4):369-74. doi: 10.1016/S0377-1237(05)80071-1. Epub 2011 Jul 21 [PubMed PMID: 27407812]
Dwilow R, Fanella S. Invasive meningococcal disease in the 21st century—an update for the clinician. Current neurology and neuroscience reports. 2015 Mar:15(3):2. doi: 10.1007/s11910-015-0524-6. Epub [PubMed PMID: 25637287]
Heckenberg SGB, de Gans J, Brouwer MC, Weisfelt M, Piet JR, Spanjaard L, van der Ende A, van de Beek D. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Medicine. 2008 Jul:87(4):185-192. doi: 10.1097/MD.0b013e318180a6b4. Epub [PubMed PMID: 18626301]
Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, Harnden A, Mant D, Levin M. Clinical recognition of meningococcal disease in children and adolescents. Lancet (London, England). 2006 Feb 4:367(9508):397-403 [PubMed PMID: 16458763]
Verghese A, Gallemore G. Kernig's and Brudzinski's signs revisited. Reviews of infectious diseases. 1987 Nov-Dec:9(6):1187-92 [PubMed PMID: 3321367]
Tacon CL, Flower O. Diagnosis and management of bacterial meningitis in the paediatric population: a review. Emergency medicine international. 2012:2012():320309. doi: 10.1155/2012/320309. Epub 2012 Sep 20 [PubMed PMID: 23050153]
El Bashir H, Laundy M, Booy R. Diagnosis and treatment of bacterial meningitis. Archives of disease in childhood. 2003 Jul:88(7):615-20 [PubMed PMID: 12818910]
Bryant PA, Li HY, Zaia A, Griffith J, Hogg G, Curtis N, Carapetis JR. Prospective study of a real-time PCR that is highly sensitive, specific, and clinically useful for diagnosis of meningococcal disease in children. Journal of clinical microbiology. 2004 Jul:42(7):2919-25 [PubMed PMID: 15243039]
Bourke TW, Fairley DJ, McKenna JP, Coyle PV, Shields MD. Clinical Evaluation of Streptococcus pneumoniae Polymerase Chain Reaction in Children with Suspected Septicemia. The Pediatric infectious disease journal. 2015 Nov:34(11):1276-7. doi: 10.1097/INF.0000000000000877. Epub [PubMed PMID: 26457903]
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic acids research. 2000 Jun 15:28(12):E63 [PubMed PMID: 10871386]
Pollard AJ, Nadel S, Ninis N, Faust SN, Levin M. Emergency management of meningococcal disease: eight years on. Archives of disease in childhood. 2007 Apr:92(4):283-6 [PubMed PMID: 17376933]
Crosswell JM, Nicholson WR, Lennon DR. Rapid sterilisation of cerebrospinal fluid in meningococcal meningitis: Implications for treatment duration. Journal of paediatrics and child health. 2006 Apr:42(4):170-3 [PubMed PMID: 16630316]
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004 Nov 1:39(9):1267-84 [PubMed PMID: 15494903]
Level 1 (high-level) evidenceGrubbauer HM, Dornbusch HJ, Dittrich P, Weippl G, Mutz I, Zobel G, Georgopoulos A, Fotter R. Ceftriaxone monotherapy for bacterial meningitis in children. Chemotherapy. 1990:36(6):441-7 [PubMed PMID: 2292206]
Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet (London, England). 2007 Jun 30:369(9580):2196-2210. doi: 10.1016/S0140-6736(07)61016-2. Epub [PubMed PMID: 17604802]
Gardner P. Clinical practice. Prevention of meningococcal disease. The New England journal of medicine. 2006 Oct 5:355(14):1466-73 [PubMed PMID: 17021322]
Runde TJ,Hafner JW, Meningitis, Bacterial 2019 Jan; [PubMed PMID: 29261975]
. Analysis of endemic meningococcal disease by serogroup and evaluation of chemoprophylaxis. The Journal of infectious diseases. 1976 Aug:134(2):201-4 [PubMed PMID: 823273]
Hersi K, Gonzalez FJ, Kondamudi NP. Meningitis. StatPearls. 2024 Jan:(): [PubMed PMID: 29083833]
Nadel S, Kroll JS. Diagnosis and management of meningococcal disease: the need for centralized care. FEMS microbiology reviews. 2007 Jan:31(1):71-83 [PubMed PMID: 17233636]
Cartwright K, Reilly S, White D, Stuart J. Early treatment with parenteral penicillin in meningococcal disease. BMJ (Clinical research ed.). 1992 Jul 18:305(6846):143-7 [PubMed PMID: 1515827]
van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. The New England journal of medicine. 2004 Oct 28:351(18):1849-59 [PubMed PMID: 15509818]
Solnick JV. Treatment with moxifloxacin versus standard therapy for community-acquired pneumonia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006 Jul 1:43(1):109; author reply 110-1 [PubMed PMID: 16758427]
Level 3 (low-level) evidenceBloch D, Murray K, Peterson E, Ngai S, Rubinstein I, Halse TA, Ezeoke I, Miller L, Arakaki L, Ramautar A, Antwi M, Del Rosso P, Dorsinville M, Clark S, Halbrook M, Kennedy J, Braunstein S, Weiss D. Sex Difference in Meningococcal Disease Mortality, New York City, 2008-2016. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Aug 16:67(5):760-769. doi: 10.1093/cid/ciy183. Epub [PubMed PMID: 29509877]
Hoffman O, Weber RJ. Pathophysiology and treatment of bacterial meningitis. Therapeutic advances in neurological disorders. 2009 Nov:2(6):1-7. doi: 10.1177/1756285609337975. Epub [PubMed PMID: 21180625]
Level 3 (low-level) evidenceCrum-Cianflone N, Sullivan E. Meningococcal Vaccinations. Infectious diseases and therapy. 2016 Jun:5(2):89-112. doi: 10.1007/s40121-016-0107-0. Epub 2016 Apr 16 [PubMed PMID: 27086142]
Mitchell GK, Tieman JJ, Shelby-James TM. Multidisciplinary care planning and teamwork in primary care. The Medical journal of Australia. 2008 Apr 21:188(S8):S61-4 [PubMed PMID: 18429739]