 Cardiac Manifestations of Coronavirus (COVID-19)
Cardiac Manifestations of Coronavirus (COVID-19)
Introduction
Coronaviruses are a large family of single-stranded positive-sense, enveloped RNA viruses that can infect many animal species. Human coronaviruses can be divided based on their pathogenicity. The types with high pathogenicity include SARS-CoV, MERS-CoV, and the current novel SARS-CoV2 viruses.[1]
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections were first identified in December 2019 and termed coronavirus disease 2019 (COVID-19). By March 2020, it was declared a global pandemic by the World Health Organization (WHO).[2]
Although primarily a respiratory disease, COVID-19 has been associated with many cardiac complications. Cardiac injury is recognized as one of the most frequent complications of the disease.[3] Long-term cardiac complications following COVID-19 include ischemic heart disease, heart failure, arrhythmias, and myocarditis.[3]
Studies have consistently shown that underlying cardiovascular disease in patients with COVID-19 and/or the development of acute cardiac injury due to COVID-19 illness is associated with significantly worse outcomes.[4] This activity reviews the clinical manifestations of cardiac complications related to COVID-19 infections and outlines their recommended evaluation as well as their treatment.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Coronaviruses comprise a family within the order Nidovirales.[5] They consist of four genera:
- Alphacoronavirus
- Betacoronavirus
- Bammacoronavirus
- Deltacoronavirus.
Coronaviruses are common in birds and mammals. They are ubiquitous and are among the most common causes of community-acquired upper respiratory tract infections.[5] They mainly cause mild, self-limiting illnesses and are transmitted by respiratory aerosol/droplets. The virus responsible for the COVID-19 outbreak is called SARS-CoV-2, which is a betacoronavirus and has 76.4% amino acid sequence homology in its spike protein compared to the SARS-CoV virus that caused the 2003 outbreak in China.[5]
Morphologically it is a positive-sense single-stranded RNA virus with an approximately 30 kb genome that transcribes four structural proteins (surface (S), envelope (E), membrane (M), and nucleocapsid (N)) and some accessory proteins. Nonstructural proteins involved in replication and transcription are also present. The spike proteins facilitate entry into host cells by binding to angiotensin-converting enzyme 2 (ACE-2) receptors.[5]
Although the respiratory system is the main site of entry and infection in humans, the presence of ACE-2 receptors in other organ systems may lead to direct injury at these sites. In humans, the ACE-2 receptors are abundantly present in the lung type II alveolar cells, enterocytes of the gastrointestinal tract, endothelial cells, smooth muscle cells, cortical neurons, and glial cells.[5] As the virus replicates, it can enter cardiac cells and cause direct injury, which results in cardiac manifestations such as myocarditis.[6] However, clinical data has not consistently supported direct cardiomyocyte death secondary to cardiomyocyte infection.[7]
Alternate mechanisms include overwhelming systemic inflammation (the cytokine storm), which results in increased oxygen demand with ongoing hypoxia, coagulopathy, and arrhythmias.[6] This is most concerning for those with underlying cardiovascular disease, who may have little physiologic reserve for this stress. Clinical data shows a substantial association between preexisting cardiovascular disease (e.g., hypertension and coronary artery disease) and the risk of severe COVID-19 illness.[8] Clear pathogenic causes of this association have not been elucidated; however, possible causes include impaired physiologic reserve, augmented inflammatory response, and SARS-CoV-2-induced endothelial dysfunction.[9]
Many studies have shown that in hospitalized patients with COVID-19, preexisting cardiovascular disease and cardiac injury predict higher mortality.[9] According to the United States Centers for Disease Control and Prevention (CDC), cardiovascular diseases such as heart failure, coronary artery disease, and cardiomyopathies confer a higher risk for severe COVID-19 disease and associated poor outcomes with "conclusive data" supporting this association. In a large case series of 5700 patients with COVID-19 infection admitted to 12 hospitals in New York, the prevalence of hypertension, diabetes, and coronary artery disease was 57%, 34%, and 11%, respectively.[10] A meta-analysis from 2020 reported that patients with chronic heart disease are more likely to have acute cardiovascular events when infected with SARS-CoV-2.[11]
Advanced age is another risk factor for cardiac injury following SARS-CoV-2 infection. A meta-analysis of 21 studies (over 6000 patients) reported a two-fold higher incidence of cardiac injury in patients older than 60 years compared to younger cohorts admitted with a COVID-19 infection.[12]
Epidemiology
Cardiovascular complications, as evidenced by acute cardiac injury, new-onset systolic heart failure, pericardial effusion, and acute myocarditis, have been reported in hospitalized patients since the beginning of the pandemic. A meta-analysis from 2021 reported a cardiac injury prevalence of 22% among all hospitalized patients with COVID-19.[12] In patients with severe COVID-19 illness, they reported that 28% had documented cardiac injury. In patients older than 60 years, the prevalence of COVID-19-associated cardiac injury rose to 30%.[12]
Common cardiac complications of COVID-19 include acute coronary syndrome and arrhythmias.[3] These tend to occur in older patients with known cardiovascular risk factors such as hypertension, diabetes mellitus, and coronary artery disease. Myocardial infarction has been noted in up to 1.3% of all COVID-19 patients.[13] The prevalence of myocardial infarctions in patients who died from COVID-19 illness rises to 4.9%.[13]
Arrhythmias are much more common by comparison. They have been reported in up to 10.4% of patients affected with moderate to severe COVID-19 illness.[13] The most commonly noted arrhythmia in these patients is atrial fibrillation.
Congestive heart failure is reported in 2.8% of patients with COVID-19 illness, with an increase to 24% in patients who died from this infection.[13]
Acute myopericarditis is reported more frequently in younger patients without any pre-existing cardiovascular risk factors.[3] Still, the incidence of de novo cardiac complications in young patients is low.[3] COVID-19–associated acute myocarditis has been shown to occur at a rate of 2 to 4 per 1000 hospitalizations and can occur with or without concomitant pneumonia.[7] However, given the risk for mortality, it warrants immediate attention if signs and symptoms suggest cardiac injury.[3]
Pathophysiology
Multiple mechanisms have been suggested as the cause of cardiac damage; however, true pathogenic mechanisms have not been clearly elucidated thus far. Possible causes include cytokine-mediated damage, microvascular thrombi, and/or direct cardiomyocyte injury due to viral invasion of the myocardium.[14]
Catecholamine-induced microvascular dysfunction due to the intense inflammatory state caused by COVID-19 is thought to cause COVID-19-associated Takosubo cardiomyopathy.[14]
Autopsy evaluations have revealed small vessel endothelial damage and vasculitis (neutrophilic) as the cause of multisystem inflammatory syndrome after a COVID-19 infection in one case report.[15] They reported that the "cardiac myocytes did not seem to be the target of the inflammatory process." Other post-mortem studies revealed similar findings with endotheliitis in several organs and evidence of inflammatory cell death.[16] These studies also noted the presence of viral bodies in affected organs and host inflammatory response leading to endothelial damage.[16]
Pre-existing cardiovascular disease is associated with chronic activation of inflammatory pathways, which may act synergistically with COVID-19-induced inflammation.[9] Pre-existing inflammation and superimposed endothelial dysfunction can accelerate coronary plaque instability, which has been proposed as a cause of cardiovascular complications in patients with COVID-19 illness.[9]
Additionally, patients with known cardiovascular disease have limited physiologic reserves, which may predispose these patients to cardiovascular damage in the setting of exogenous stress.[9]
Histopathology
Although the exact pathogenic mechanisms remain to be elucidated, post-mortem analysis can help provide a preliminary understanding of cardiovascular complications associated with this disease. Post-mortem analyses revealed myocardial necrosis and myocardial edema as the most common finding.[17] In this study, the most prevalent chronic changes were myocardial hypertrophy, coronary artery disease, and fibrosis. A higher prevalence of cardiac amyloidosis among those deceased due to COVID-19 illness was also noted, suggesting that it may predispose patients to poorer outcomes.[17]
A review of thrombotic complications in these patients revealed a prevalence of 36.2% for microvessel thrombi, 22.2% for pulmonary embolism, and 11.8% for acute myocardial infarction.[17] Microvascular thrombosis likely plays a significant role in cardiac complications of this disease, which is cited as the reason why a culprit lesion is not identified in more than 40% of patients who undergo coronary angiography when suspected to have acute myocardial infarction.[17]
History and Physical
Patients who go on to develop cardiac complications of acute COVID-19 illness present with a variety of symptoms. Most have typical symptoms of COVID-19 infection, such as cough, fever, and dyspnea, while some are asymptomatic—only a minority of patients present with symptoms suggestive of heart disease. Initial epidemiologic studies revealed a low frequency of cardiac symptoms as initial presentations of COVID-19 illness, with one study stating that 7.3% of the patients presented with palpitations and another stating that only 2% of the patients presented with chest pain.[18][19] The vast majority of the patients will present with cough, fever, myalgias, and headache, which are typical symptoms of COVID-19 illness.[20]
Critically ill patients warrant close monitoring of their cardiac status; however, these patients likely manifest cardiac injury as a function of their severe illness. Consequently, typical symptoms of acute coronary syndrome are seldom seen. A retrospective study from November 2020 reported that the rate of any myocardial injury was similar between patients with COVID-19 illness and those with acute respiratory distress syndrome (ARDS) from non-COVID-19 related etiologies.[21]
The authors also concluded that myocardial injury in critically ill COVID-19 patients was reflective of baseline risk and comorbidities as well as the underlying multisystem organ dysfunction due to their acute illness. In their study, 50% of the patients with critical COVID-19 illness who required mechanical ventilation had myocardial injury based on serum markers. However, high rates of myocardial injury were seen in non–COVID-19–related critical illnesses as well. They concluded that myocardial injury in severe COVID-19 was similar to that of the general ARDS cohort.[21]
Patients who develop arrhythmias, acute coronary syndrome, or myocarditis present with signs and symptoms that are typical for these diagnoses, without any unique identifiable presentations associated with COVID-19. Acute heart failure in COVID-19 has an incidence reported around 23%.[22] The majority of these patients will have an elevated cardiac biomarker as well. A small portion of these patients will have Takotsubo syndrome.[22]
Evaluation
Cardiac biomarkers such as troponin and B-type natriuretic peptide (BNP) are usually elevated in these patients. They may identify those with cardiac injury due to COVID-19 critical illness in the absence of symptoms.[3] An electrocardiogram and echocardiography are warranted in evaluating patients suspected of having a myocardial injury to differentiate those with acute myocardial infarction due to atherothrombotic disease from those with demand ischemia. Specific cardiac testing is seldom warranted.
In patients with post-COVID-19 syndrome and persistent symptoms, however, cardiac testing may help identify those needing cardiopulmonary rehabilitation.[23] Results of cardiopulmonary testing in patients seen in a post-COVID-19 care clinic due to persistent symptoms of fatigue, myalgias, and dyspnea reported decreased peak oxygen uptake in these patients.[23] Those with persistent symptoms were noted to have mean peak oxygen uptake 30% below predicted, with less than 10% of these patients having a normal value.[23]
In acutely ill patients, elevated cardiac markers, especially troponin levels, must be taken in context to prevent erroneous diagnoses. A retrospective study from 2022 stated that of the 2152 patients studied, 88% had high-sensitivity troponin-T testing, with 57% having a value above the 99th percentile. Further analysis of those with elevated levels revealed that only 47% of the patients had a primary cardiac etiology for the elevated troponin level.[22]
This study outlines the need for appropriate testing to determine the cause of elevated cardiac markers in these patients. The authors also noted that all-cause mortality in patients with a primary cardiac etiology was higher at 28% compared to those with an elevated troponin level without a cardiac etiology (16%). Patients who did not have an elevated troponin level had all-cause mortality of 3.4% in this study.[22]
Electrocardiograms are essential for evaluating all critically ill patients presenting to the hospital and those with a possible myocardial injury secondary to an underlying infection. Electrocardiographic findings in patients who present to the hospital with COVID-19 disease include tachycardia with a mean heart rate of 90 +/- 19 bpm and a mean Bazett-corrected QT interval of 449 +/- 144 ms.[24]
Most patients have normal sinus rhythm. Atrial fibrillation/flutter was the most common at 5.6% when arrhythmias were present. Abnormal intraventricular conduction was noted in 11.8%, with right bundle branch block in 7.8% of the patients, left bundle branch block in 1.5%, and nonspecific intraventricular block in 2.5% of the patients.[24] In this study, a previous Q-wave myocardial infarction was present in 13.9% of the patients. According to one study, approximately 8.4% of patients reported having a new-onset tachyarrhythmia or block during the index hospitalization for acute COVID-19 illness.[22]
Echocardiography is an important diagnostic test in patients with electrographic or serum biomarker evidence of cardiac injury. A higher prevalence of major echocardiographic abnormalities is noted in patients with myocardial injury as a result of acute COVID-19 illness.[25] Common findings include left ventricular (LV) wall motion abnormalities, global LV dysfunction, LV diastolic dysfunction, right ventricular (RV) dysfunction, and pericardial effusions.[25]
However, as stated before, elevated cardiac biomarkers are not predictive of echocardiographic abnormalities. One study reported that most patients who underwent echocardiography with elevated troponin levels during an acute COVID-19 illness hospitalization had an LV ejection fraction (EF) greater than 50%.[22] This study reported wall motion abnormalities in only 22% of the patients. Right ventricular (RV) dysfunction was detected in 17% of the patients, while pericardial effusion was observed in only 6.6% of the patients.[22]
The significance of obtaining an echocardiogram in patients with elevated serum cardiac biomarkers is supported by the evidence that myocardial injury correlates with higher mortality if there are echocardiographic abnormalities. According to one study, myocardial injury/cardiac biomarker elevation in the absence of echocardiographic abnormalities did not predict higher mortality.[25] The presence of RV dysfunction or LV wall motion abnormalities in more than 50% of segments is an independent predictor of mortality.[22]
A diagnosis of myocarditis requires tissue sampling. Radiographic imaging, such as a cardiac magnetic resonance image (MRI), suggests a high number of patients have ongoing myocardial inflammation after a COVID-19 infection; however, this is not diagnostic. Moreover, autopsy analysis suggests that clinical criteria for diagnosing myocarditis (electrocardiograms and cardiac biomarkers) overestimate the true incidence of myocarditis.[22] Postmortem cardiac examinations reveal a histologically confirmed prevalence of myocarditis at 0.5%.[22]
Treatment / Management
Cardiac care needs to be optimized for COVID-19 patients with an aim for early detection and management of cardiac ailments with the simultaneous aim of triaging cases and proper protection to prevent or minimize COVID-19 exposure.[26]
The optimal management for myocardial injury in patients with acute COVID-19 illness is not defined. In the absence of guidelines, patients with demand ischemia and clinically suspected myocarditis are usually treated with supportive care, appropriate management of hemodynamics, and symptom control. Patients with COVID-19-associated heart failure or those who are found to have LV systolic dysfunction after this infection should receive standard goal-directed guideline therapy for congestive heart failure. Patients with arrhythmias should receive pharmacologic and/or surgical/ablative interventions as indicated based on their conduction or rhythm abnormality.
Early in 2020, a controversy grew regarding the safe usage of ACE inhibitors (ACEI) and angiotensin receptor-blocking (ARB) drugs in patients infected with COVID-19. The current consensus agrees to the continued use of these medications.[27][28] The BRACE CORONA trial found no significant difference in the number of days alive and out of hospital through 30 days among subjects receiving continuous ACEI/ARB and hospitalized for COVID-19 compared to those in whom these medicines were temporarily suspended.[29]
According to the latest United States National Institutes of Health guidelines, ACEI and ARB therapy should be continued for their underlying cardiac indication unless the acute status warrants withholding the medication (such as acute renal failure). The American Heart Association, the Heart Failure Society of America, and the American College of Cardiology issued a joint statement stating that renin-angiotensin-aldosterone system antagonists should not be added or removed for a diagnosis of COVID-19 infection.[28]
Instead, the clinical decision regarding these agents should be based on the individual hemodynamic and clinical condition. If the patient is on these agents for underlying cardiac/vascular conditions, they should be continued as prescribed in those with COVID-19.
In patients with post-COVID syndrome and deficits in cardiopulmonary testing, physical and/or occupational therapy with subsequent graded exercise programs is advised.[23] Supervised exercise in cardiopulmonary rehabilitation programs is preferred in the initial phase.[23]
Differential Diagnosis
The differential diagnosis of acute myocardial injury in patients with COVID-19 infection includes several conditions.[22] These include:
- Chronic troponin elevation
- Pulmonary embolism
- Noncardiogenic shock
- Sepsis
- Renal failure
Prognosis
As stated above, cardiac injury in patients with acute COVID-19 illness is associated with an increased risk of all-cause mortality.[12] Even in patients without a primary cardiac cause of elevated cardiac biomarkers in acute COVID-19 disease, the mortality rate is higher than those without an elevation of these markers (16% versus 3.4%).[22]
The prognostic significance of cardiovascular disease was amply illustrated in a cohort of 191 patients in which 30% had hypertension and constituted 48% of nonsurvivors, whereas 8% had cardiovascular disease and constituted 13% of nonsurvivors.[30]
In a report of 44672 confirmed cases of COVID-19 from the Chinese Center for Disease Control and Prevention, the overall case-fatality rate was 2.3% for the entire cohort but significantly higher for patients with hypertension (6%), diabetes (7%), or cardiovascular disease (11%).[31]
COVID-19-associated acute myocarditis is frequently complicated by shock (38.9%).[7] However, isolated myocarditis has a better prognosis than myocarditis with concomitant pneumonia due to COVID-19.[7]
Complications
The United States Department of Veterans Affairs (VA) database demonstrated an excess burden of cerebrovascular disorders, arrhythmia, ischemic heart disease, heart failure, and thrombotic disorders after COVID-19 infection.[13] Although these observations were true for those not hospitalized for acute COVID-19 infection, the most significant burden of these complications was seen in patients requiring intensive care. Atrial fibrillation and heart failure contributed the most to this excess burden, occurring in more than 10 additional individuals per 1,000 persons.[13]
The burden of disease stretched beyond the acute phase as well. New-onset hypertension and heart failure were present in 2% of patients who were more than one year out from their acute COVID-19 infection.[13] New right-sided heart failure in the absence of hypertension or left heart failure was also seen in 2.7% of these patients.
Acute myocardial injury is also associated with ongoing symptoms 12 months after the initial COVID-19 infection, with an increased hospital readmission rate.[13] This is likely because myocardial dysfunction persists after the initial infection. Short-term follow-up (2 to 3 months after the initial infection) revealed 78% of patients had abnormalities on cardiac magnetic resonance imaging (CMR). Studies using echocardiography to assess RV function after recovery from severe COVID revealed RV longitudinal strain in 42% of patients. However, they did not reveal overt RV dysfunction by tricuspid annular plane systolic excursion (TAPSE) or RV fractional shortening.[13]
Deterrence and Patient Education
Prevention of COVID-19 infection is the primary preventive strategy to decrease associated cardiac complications. COVID-19 vaccines were shown to be highly effective in reducing COVID-19-associated hospitalizations, ICU admissions, and emergency department visits.[32]
Although their efficacy is low in the elderly population and those with a high number of comorbid conditions, they still maintain high efficacy in protection against severe disease and death.[33] Unvaccinated adults are more likely to be hospitalized compared with vaccinated adults.[34]
Enhancing Healthcare Team Outcomes
Cardiac complications of COVID-19 illness comprise a heterogeneous mix of conditions that may be cardiac or noncardiac. Regardless of the cause, these conditions are associated with an increased burden of illness and excess mortality. Although they are primarily seen in patients with underlying cardiac conditions and/or advanced age, they may also be seen in those without preexisting cardiac disease. Understanding the significance of these conditions in association with COVID-19 illness is of critical importance in ensuring an accurate diagnosis and timely management.
Clinicians and other interprofessional healthcare team members need to maintain a high degree of suspicion for developing these complications, especially since typical symptoms of cardiac disease may not be present in these patients. The clinical nurse is of prime importance in monitoring the cardiac rhythms and rates of patients admitted with an acute COVID-19 infection to ensure these complications are caught early and addressed appropriately. Clinical pharmacists are essential in helping providers manage patients with guideline-recommended therapies when complications such as arrhythmias and/or heart failure develop. Both nurses and pharmacists must be empowered to alert clinicians regarding any change in patient status or other concerns they may have.
It is also important to remember that patients may have residual dysfunction for many months after the acute illness. Most will benefit from cardiopulmonary rehabilitation with supervised physical and occupational therapy to optimize their progress. A well-integrated interprofessional team of clinical providers can significantly optimize the outcomes of patients affected by cardiac complications of an acute COVID-19 infection. [Level 5]
Media
(Click Image to Enlarge)
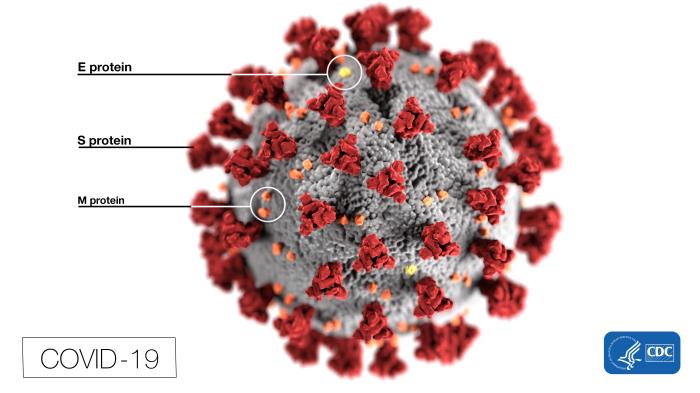
Structure of COVID-19. This illustration, created by the Centers for Disease Control and Prevention (CDC), reveals ultrastructural morphology exhibited by coronaviruses. Note the spikes that adorn the virus's outer surface, which impart the look of a corona surrounding the virion when viewed electron microscopically. In this view, the protein particles E, S, and M, also located on the particle's outer surface, have all been labeled as well. A novel coronavirus, SARS-CoV-2, was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China, in 2019. The infection caused by this virus has been named COVID-19.
Contributed from the CDC, Alissa Eckert, MS; Dan Higgins, MAM (Public Domain)
References
Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiology and molecular biology reviews : MMBR. 2005 Dec:69(4):635-64 [PubMed PMID: 16339739]
Level 3 (low-level) evidenceMallah SI, Ghorab OK, Al-Salmi S, Abdellatif OS, Tharmaratnam T, Iskandar MA, Sefen JAN, Sidhu P, Atallah B, El-Lababidi R, Al-Qahtani M. COVID-19: breaking down a global health crisis. Annals of clinical microbiology and antimicrobials. 2021 May 18:20(1):35. doi: 10.1186/s12941-021-00438-7. Epub 2021 May 18 [PubMed PMID: 34006330]
Srinivasan A, Wong F, Couch LS, Wang BX. Cardiac Complications of COVID-19 in Low-Risk Patients. Viruses. 2022 Jun 17:14(6):. doi: 10.3390/v14061322. Epub 2022 Jun 17 [PubMed PMID: 35746793]
Bansal M. Cardiovascular disease and COVID-19. Diabetes & metabolic syndrome. 2020 May-Jun:14(3):247-250. doi: 10.1016/j.dsx.2020.03.013. Epub 2020 Mar 25 [PubMed PMID: 32247212]
Zapor M. Persistent Detection and Infectious Potential of SARS-CoV-2 Virus in Clinical Specimens from COVID-19 Patients. Viruses. 2020 Dec 3:12(12):. doi: 10.3390/v12121384. Epub 2020 Dec 3 [PubMed PMID: 33287245]
Satterfield BA, Bhatt DL, Gersh BJ. Cardiac involvement in the long-term implications of COVID-19. Nature reviews. Cardiology. 2022 May:19(5):332-341. doi: 10.1038/s41569-021-00631-3. Epub 2021 Oct 22 [PubMed PMID: 34686843]
Ammirati E, Lupi L, Palazzini M, Hendren NS, Grodin JL, Cannistraci CV, Schmidt M, Hekimian G, Peretto G, Bochaton T, Hayek A, Piriou N, Leonardi S, Guida S, Turco A, Sala S, Uribarri A, Van de Heyning CM, Mapelli M, Campodonico J, Pedrotti P, Barrionuevo Sánchez MI, Ariza Sole A, Marini M, Matassini MV, Vourc'h M, Cannatà A, Bromage DI, Briguglia D, Salamanca J, Diez-Villanueva P, Lehtonen J, Huang F, Russel S, Soriano F, Turrini F, Cipriani M, Bramerio M, Di Pasquale M, Grosu A, Senni M, Farina D, Agostoni P, Rizzo S, De Gaspari M, Marzo F, Duran JM, Adler ED, Giannattasio C, Basso C, McDonagh T, Kerneis M, Combes A, Camici PG, de Lemos JA, Metra M. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation. 2022 Apr 12:145(15):1123-1139. doi: 10.1161/CIRCULATIONAHA.121.056817. Epub 2022 Apr 11 [PubMed PMID: 35404682]
Figliozzi S, Masci PG, Ahmadi N, Tondi L, Koutli E, Aimo A, Stamatelopoulos K, Dimopoulos MA, Caforio ALP, Georgiopoulos G. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. European journal of clinical investigation. 2020 Oct:50(10):e13362. doi: 10.1111/eci.13362. Epub 2020 Aug 27 [PubMed PMID: 32726868]
Level 1 (high-level) evidenceChatterjee NA, Cheng RK. Cardiovascular disease and COVID-19: implications for prevention, surveillance and treatment. Heart (British Cardiac Society). 2020 Aug:106(15):1119-1121. doi: 10.1136/heartjnl-2020-317110. Epub 2020 May 25 [PubMed PMID: 32451360]
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020 May 26:323(20):2052-2059. doi: 10.1001/jama.2020.6775. Epub [PubMed PMID: 32320003]
Level 2 (mid-level) evidenceZheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. The Journal of infection. 2020 Aug:81(2):e16-e25. doi: 10.1016/j.jinf.2020.04.021. Epub 2020 Apr 23 [PubMed PMID: 32335169]
Level 1 (high-level) evidenceFu L, Liu X, Su Y, Ma J, Hong K. Prevalence and impact of cardiac injury on COVID-19: A systematic review and meta-analysis. Clinical cardiology. 2021 Feb:44(2):276-283. doi: 10.1002/clc.23540. Epub 2020 Dec 31 [PubMed PMID: 33382482]
Level 1 (high-level) evidenceTobler DL, Pruzansky AJ, Naderi S, Ambrosy AP, Slade JJ. Long-Term Cardiovascular Effects of COVID-19: Emerging Data Relevant to the Cardiovascular Clinician. Current atherosclerosis reports. 2022 Jul:24(7):563-570. doi: 10.1007/s11883-022-01032-8. Epub 2022 May 4 [PubMed PMID: 35507278]
Giustino G, Croft LB, Oates CP, Rahman K, Lerakis S, Reddy VY, Goldman M. Takotsubo Cardiomyopathy in COVID-19. Journal of the American College of Cardiology. 2020 Aug 4:76(5):628-629. doi: 10.1016/j.jacc.2020.05.068. Epub 2020 Jun 8 [PubMed PMID: 32517962]
Fox SE, Lameira FS, Rinker EB, Vander Heide RS. Cardiac Endotheliitis and Multisystem Inflammatory Syndrome After COVID-19. Annals of internal medicine. 2020 Dec 15:173(12):1025-1027. doi: 10.7326/L20-0882. Epub 2020 Jul 29 [PubMed PMID: 32726150]
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England). 2020 May 2:395(10234):1417-1418. doi: 10.1016/S0140-6736(20)30937-5. Epub 2020 Apr 21 [PubMed PMID: 32325026]
Almamlouk R, Kashour T, Obeidat S, Bois MC, Maleszewski JJ, Omrani OA, Tleyjeh R, Berbari E, Chakhachiro Z, Zein-Sabatto B, Gerberi D, Tleyjeh IM, Cardiac Autopsy in COVID-19 Study Group, Paniz Mondolfi AE, Finn AV, Duarte-Neto AN, Rapkiewicz AV, Frustaci A, Keresztesi AA, Hanley B, Märkl B, Lardi C, Bryce C, Lindner D, Aguiar D, Westermann D, Stroberg E, Duval EJ, Youd E, Bulfamante GP, Salmon I, Auer J, Maleszewski JJ, Hirschbühl K, Absil L, Barton LM, Ferraz da Silva LF, Moore L, Dolhnikoff M, Lammens M, Bois MC, Osborn M, Remmelink M, Nascimento Saldiva PH, Jorens PG, Craver R, Aparecida de Almeida Monteiro R, Scendoni R, Mukhopadhyay S, Suzuki T, Mauad T, Fracasso T, Grimes Z. COVID-19-Associated cardiac pathology at the postmortem evaluation: a collaborative systematic review. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2022 Aug:28(8):1066-1075. doi: 10.1016/j.cmi.2022.03.021. Epub 2022 Mar 23 [PubMed PMID: 35339672]
Level 1 (high-level) evidenceLiu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chinese medical journal. 2020 May 5:133(9):1025-1031. doi: 10.1097/CM9.0000000000000744. Epub [PubMed PMID: 32044814]
Level 3 (low-level) evidenceChen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020 Feb 15:395(10223):507-513. doi: 10.1016/S0140-6736(20)30211-7. Epub 2020 Jan 30 [PubMed PMID: 32007143]
Level 2 (mid-level) evidenceStokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR. Morbidity and mortality weekly report. 2020 Jun 19:69(24):759-765. doi: 10.15585/mmwr.mm6924e2. Epub 2020 Jun 19 [PubMed PMID: 32555134]
Level 3 (low-level) evidenceMetkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, Michos ED, Nolley EP, Post WS, Resar JR, Thiemann DR, Trost JC, Hasan RK. Myocardial Injury in Severe COVID-19 Compared With Non-COVID-19 Acute Respiratory Distress Syndrome. Circulation. 2021 Feb 9:143(6):553-565. doi: 10.1161/CIRCULATIONAHA.120.050543. Epub 2020 Nov 13 [PubMed PMID: 33186055]
Khaloo P, Shaqdan A, Ledesma PA, Uzomah UA, Galvin J, Ptaszek LM, Ruskin JN. Distinct etiologies of high-sensitivity troponin T elevation predict different mortality risks for patients hospitalized with COVID-19. International journal of cardiology. 2022 Mar 15:351():118-125. doi: 10.1016/j.ijcard.2021.12.029. Epub 2021 Dec 21 [PubMed PMID: 34952038]
Level 2 (mid-level) evidenceSmer A, Squires RW, Bonikowske AR, Allison TG, Mainville RN, Williams MA. Cardiac Complications of COVID-19 Infection and the Role of Physical Activity. Journal of cardiopulmonary rehabilitation and prevention. 2023 Jan 1:43(1):8-14. doi: 10.1097/HCR.0000000000000701. Epub 2022 Jul 15 [PubMed PMID: 35839441]
McCullough SA, Goyal P, Krishnan U, Choi JJ, Safford MM, Okin PM. Electrocardiographic Findings in Coronavirus Disease-19: Insights on Mortality and Underlying Myocardial Processes. Journal of cardiac failure. 2020 Jul:26(7):626-632. doi: 10.1016/j.cardfail.2020.06.005. Epub 2020 Jun 13 [PubMed PMID: 32544622]
Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, Danilov T, Kukar N, Shaban N, Kini A, Camaj A, Bienstock SW, Rashed ER, Rahman K, Oates CP, Buckley S, Elbaum LS, Arkonac D, Fiter R, Singh R, Li E, Razuk V, Robinson SE, Miller M, Bier B, Donghi V, Pisaniello M, Mantovani R, Pinto G, Rota I, Baggio S, Chiarito M, Fazzari F, Cusmano I, Curzi M, Ro R, Malick W, Kamran M, Kohli-Seth R, Bassily-Marcus AM, Neibart E, Serrao G, Perk G, Mancini D, Reddy VY, Pinney SP, Dangas G, Blasi F, Sharma SK, Mehran R, Condorelli G, Stone GW, Fuster V, Lerakis S, Goldman ME. Characterization of Myocardial Injury in Patients With COVID-19. Journal of the American College of Cardiology. 2020 Nov 3:76(18):2043-2055. doi: 10.1016/j.jacc.2020.08.069. Epub [PubMed PMID: 33121710]
Lakkireddy DR, Chung MK, Gopinathannair R, Patton KK, Gluckman TJ, Turagam M, Cheung JW, Patel P, Sotomonte J, Lampert R, Han JK, Rajagopalan B, Eckhardt L, Joglar J, Sandau KE, Olshansky B, Wan E, Noseworthy PA, Leal M, Kaufman E, Gutierrez A, Marine JE, Wang PJ, Russo AM. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart rhythm. 2020 Sep:17(9):e233-e241. doi: 10.1016/j.hrthm.2020.03.028. Epub 2020 Apr 1 [PubMed PMID: 32247013]
Sommerstein R, Kochen MM, Messerli FH, Gräni C. Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect? Journal of the American Heart Association. 2020 Apr 7:9(7):e016509. doi: 10.1161/JAHA.120.016509. Epub 2020 Apr 1 [PubMed PMID: 32233753]
Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. Journal of cardiac failure. 2020 May:26(5):370. doi: 10.1016/j.cardfail.2020.04.013. Epub [PubMed PMID: 32439095]
Lopes RD, Macedo AVS, de Barros E Silva PGM, Moll-Bernardes RJ, Feldman A, D'Andréa Saba Arruda G, de Souza AS, de Albuquerque DC, Mazza L, Santos MF, Salvador NZ, Gibson CM, Granger CB, Alexander JH, de Souza OF, BRACE CORONA investigators. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)--The BRACE CORONA Trial. American heart journal. 2020 Aug:226():49-59. doi: 10.1016/j.ahj.2020.05.002. Epub 2020 May 13 [PubMed PMID: 32502882]
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020 Mar 28:395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11 [PubMed PMID: 32171076]
Level 2 (mid-level) evidenceWu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7:323(13):1239-1242. doi: 10.1001/jama.2020.2648. Epub [PubMed PMID: 32091533]
Level 3 (low-level) evidenceThompson MG, Stenehjem E, Grannis S, Ball SW, Naleway AL, Ong TC, DeSilva MB, Natarajan K, Bozio CH, Lewis N, Dascomb K, Dixon BE, Birch RJ, Irving SA, Rao S, Kharbanda E, Han J, Reynolds S, Goddard K, Grisel N, Fadel WF, Levy ME, Ferdinands J, Fireman B, Arndorfer J, Valvi NR, Rowley EA, Patel P, Zerbo O, Griggs EP, Porter RM, Demarco M, Blanton L, Steffens A, Zhuang Y, Olson N, Barron M, Shifflett P, Schrag SJ, Verani JR, Fry A, Gaglani M, Azziz-Baumgartner E, Klein NP. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. The New England journal of medicine. 2021 Oct 7:385(15):1355-1371. doi: 10.1056/NEJMoa2110362. Epub 2021 Sep 8 [PubMed PMID: 34496194]
Ioannou GN, Locke ER, O'Hare AM, Bohnert ASB, Boyko EJ, Hynes DM, Berry K. COVID-19 Vaccination Effectiveness Against Infection or Death in a National U.S. Health Care System : A Target Trial Emulation Study. Annals of internal medicine. 2022 Mar:175(3):352-361. doi: 10.7326/M21-3256. Epub 2021 Dec 21 [PubMed PMID: 34928700]
Havers FP, Pham H, Taylor CA, Whitaker M, Patel K, Anglin O, Kambhampati AK, Milucky J, Zell E, Moline HL, Chai SJ, Kirley PD, Alden NB, Armistead I, Yousey-Hindes K, Meek J, Openo KP, Anderson EJ, Reeg L, Kohrman A, Lynfield R, Como-Sabetti K, Davis EM, Cline C, Muse A, Barney G, Bushey S, Felsen CB, Billing LM, Shiltz E, Sutton M, Abdullah N, Talbot HK, Schaffner W, Hill M, George A, Hall AJ, Bialek SR, Murthy NC, Murthy BP, McMorrow M. COVID-19-Associated Hospitalizations Among Vaccinated and Unvaccinated Adults 18 Years or Older in 13 US States, January 2021 to April 2022. JAMA internal medicine. 2022 Oct 1:182(10):1071-1081. doi: 10.1001/jamainternmed.2022.4299. Epub [PubMed PMID: 36074486]