Introduction
Scrub typhus is a severe infectious disease caused by the rickettsial bacterium Orientia tsutsugamushi, which displays high levels of antigenic variation.[1] The disease is a serious public health concern in the Asia-Pacific region, including, but not limited to, the region known as the "tsutsugamushi triangle." Spanning over 8 million square kilometers and affecting more than 1 billion people across countries such as Pakistan in the west, Australia in the south, and Japan in the east, this triangular area poses a significant public health threat with a high risk of fatality.[2]
A systemic review on the burden of scrub typhus in India, located within the "tsutsugamushi triangle," revealed that scrub typhus accounts for at least 25.3% of individuals with acute undifferentiated febrile illness.[3] Despite being one of the common pathogens of the cause of such encountered illness, scrub typhus remains a neglected disease in terms of research and healthcare policy formulation.
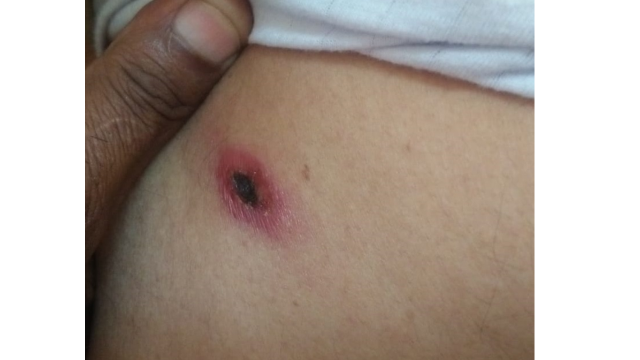
The infection is transmitted to humans through the bite of larval trombiculid mites, leading to disseminated vasculitis, perivascular inflammation, vascular leakage, and end-organ injury. Travelers to endemic regions and individuals of all ages are susceptible to this disease. Clinical features usually arise after an incubation period of 6 to 21 days and manifest as fever, headache, myalgia, and gastrointestinal symptoms. An "eschar" is a distinct characteristic of scrub typhus, which generally begins as a primary papular lesion at the site of the bite. However, it may later crust to form a black ulcer with central necrosis. However, the presence of the eschar may vary by region. The decision to initiate treatment should be based on clinical suspicion and later confirmed by serological tests.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Scrub typhus is a significant cause of underdiagnosed acute febrile illness and is prevalent in endemic regions. This disease can affect multiple organ systems primarily through vasculitis and perivascular inflammation. Mite larvae or chiggers, which carry the pathogenic gram-negative bacteria, are the vectors to humans, and they transmit the organism through their bite. The infection persists in nature through transovarial transmission in the vector and occasionally in small mammals as a reservoir.[4]
The Orientia genome exhibits a remarkably high degree of repetition, primarily due to extensive intragenomic deletions and duplications. Additionally, rearrangements involving transposable and conjugative elements within the genome further contribute to this repetitive nature. Among the structural proteins of O tsutsugamushi, the 56-kDa type-specific antigen stands out as a unique outer membrane protein. The serological characterization of this specific outer membrane protein, which exhibits variable regions, has led to the identification of several new subtypes in different geographical areas. This highlights the high genomic plasticity of Orientia tsutsugamushi beyond the previously known prototype strains such as Karp, Kato, and Gilliam.[5]
The 56-kDa type-specific antigen is also described as a primary immunogen that can elicit neutralizing antibodies during infection.[6] Due to these divergent strains of O tsutsugamushi present in different endemic countries, the development of an effective vaccine has been hindered.
Epidemiology
The available data on the epidemiology of scrub typhus are currently limited. However, prospective fever studies in Asia indicate that scrub typhus is a significant cause of non-malarial febrile illnesses. O tsutsugamushi infection is prevalent across Asia, with seroprevalence rates ranging from 9.3% to 27.9%, and there is evidence suggesting an increasing incidence. Several hypotheses have been proposed to explain the rising scrub typhus infections in endemic regions. Factors such as population growth in recent decades, increasing urbanization leading to changes in land use, and the availability of specific diagnostic tests contributing to increased case detection are significant contributors. Changes in antimicrobial prescription practices may also play a role in this trend.
In the early 1990s, a notable trend for using chloramphenicol and tetracyclines for empiric treatment of enteric fever and other acute undifferentiated fevers was apparent. These antimicrobials also act against O tsutsugamushi. However, by the late 1990s, fluoroquinolones and second and third-generation cephalosporins became more prevalent but were found to be ineffective against O tsutsugamushi. Another contributing factor may be the favorable conditions for chigger proliferation in hot and humid environments. The recent global warming trend and increased humidity during the rainy season may elevate the risk of scrub typhus infection. Changes in human behavior, such as increased participation in outdoor activities like trekking or camping, also heighten the risk of exposure to chigger vectors in their natural habitats, consequently increasing the risk of infection.[7]
Licensed vaccines are currently unavailable for scrub typhus, and systematic vector control measures have not been widely implemented.[2] Notably, O tsutsugamushi infections can affect individuals of all age groups, with no specific gender or race predilection observed.[8]
Pathophysiology
The pathophysiology of O tsutsugamushi involves vasculitis due to the infection of endothelial cells, leading to perivascular infiltration of T cells and monocytes or macrophages. Subsequently, a wide range of inflammatory responses occurs, with endothelial and non-endothelial cells producing various cytokines. These cytokines can have both beneficial effects, such as antimicrobial activity, and detrimental effects, causing tissue destruction in the infected host. This immune response's dual nature can lead to severe complications such as hepatitis, renal failure, meningoencephalitis, and respiratory failure, including acute respiratory distress syndrome (ARDS) and occasionally myocarditis.
History and Physical
In humans, the incubation period of scrub typhus typically ranges between 10 and 12 days, although it can vary from 6 to 21 days. Following the bite of the infected Leptotrombidium mite, patients often exhibit nonspecific flu-like symptoms such as fever and rash. Additionally, patients may develop an eschar at the bite site, which typically causes no pain or itching at the time of the bite (see Image. Chigger Bite Resulting in Eschar Formation). The presence of an eschar is a distinctive clinical feature of scrub typhus and occurs at the site where the chigger is fed. Initially appearing as an ulcer, the eschar enlarges and eventually undergoes central necrosis, forming a black crust. This skin lesion can resemble a burn from a cigarette.[9] Eschars commonly appear on the neck, axillary, and infraaxillary regions. In both males and females, eschars are usually observed below the umbilicus on the front of the body, and women are also commonly affected in the mammary and inframammary regions.[10]
Nonspecific symptoms such as headaches and fever are commonly observed in individuals with scrub typhus. Infected individuals may also experience generalized lymphadenopathy, nausea, vomiting, abdominal pain, and myalgia. As the disease progresses into the second week, untreated individuals may develop systemic manifestations. These can include central nervous system issues, such as acute diffuse encephalomyelitis, encephalopathy, meningitis, and, occasionally, hearing loss, cranial nerve palsies, along with various ocular manifestations.[11][12][13]
Scrub typhus meningitis is a common neurological manifestation of scrub typhus, which is characterized by headaches or nuchal rigidity along with altered sensorium or focal neurological deficits. A more severe form, known as meningoencephalitis, can occur in some patients presenting with altered sensorium and seizures. This condition is often observed alongside multiple organ dysfunction syndrome (MODS), leading to an increased risk of mortality if not promptly treated.
Cardiovascular manifestations of scrub typhus can vary from non-fatal rhythm abnormalities to signs of congestive heart failure.[9][14] The renal system may also suffer, leading to acute renal failure. Involvement of the respiratory system may manifest as interstitial pneumonia and ARDS. The gastrointestinal system may also be affected, presenting as pancreatitis, altered liver function, and diarrhea.[15][16][17]
Evaluation
Early diagnosis of acute scrub typhus infection is crucial for guiding appropriate therapy and preventing complications. In endemic areas where rapid, sensitive, and affordable diagnostic tools for scrub typhus are typically unavailable, clinicians often initiate empirical treatment based on suspicion. However, this approach can lead to misdiagnosis and subsequent mismanagement of the condition in the affected patients.
Serological methods are the mainstay for diagnosing scrub typhus. A significant immunoglobulin M (IgM) antibody titer typically emerges in primary scrub typhus infection by the end of the first week. In contrast, significant levels of IgG antibodies usually take approximately 2 weeks to develop. In cases of reinfection, IgG titers can be detectable as early as the sixth day of infection, while the levels of IgM antibody titers in such cases may vary.[1]
Serological Tests for Diagnosing Scrub Typhus
Weil-Felix testing: Although Weil-Felix testing is the oldest test still in use today, it lacks sensitivity and specificity for scrub typhus diagnosis. It relies on the cross-reactivity of the Proteus OXK strain for serodiagnosis, yet it aids in timely diagnosis despite its low sensitivity.[18]
The indirect immunofluorescent antibody: The indirect immunofluorescent antibody (IFA) test is widely regarded as the gold standard for scrub typhus diagnosis. It operates on the principle of fluorescence-labeled anti-human immunoglobulin, detecting antibodies in the patient's serum. A positive result occurs when the labeled antibody binds to the bacterial antigen on a slide. However, this method has limitations, requiring repeat testing to demonstrate the difference of a 4-fold increase in antibody titers between acute and convalescent phases, limiting its utility for guiding initial management in acute infections. Furthermore, a single high antibody titer may not be diagnostic without prior studies establishing baseline seroprevalence levels in the local population.[19]
Indirect immunoperoxidase: This test was developed to address the limitations of IFA tests. Unlike IFA, it does not require a fluorescent microscope; instead, it uses peroxidase-labeled antibodies instead of fluorescein. Indirect immunoperoxidase methods have demonstrated comparable results to IFA. However, similar to IFA, the sensitivity of this test can be influenced by various bacterial strains. Despite not needing a fluorescent microscope, technical expertise remains crucial for accurate interpretation and diagnosis.
Enzyme-linked immunoabsorbent assays: Enzyme-linked immunosorbent assays (ELISA) are the preferred method for serological diagnosis in acute scrub typhus infection due to the limited availability of resources for conducting IFA in standard laboratories, given the low sensitivity of Weil-Felix testing. ELISA utilizes a recombinant p56-kDa type-specific antigen from O tsutsugamushi strains, which binds with IgM antibodies produced against strains such as Karp, Kato, Gilliam, and TA716 during acute infections. IgM ELISA is highly sensitive and indicates a recent infection with O tsutsugamushi.[20]
Immunochromatographic tests: Immunochromatographic tests are rapid diagnostic point-of-care tests used for scrub typhus. They operate on similar principles as other serologic tests, utilizing the recombinant 56-kDa type-specific antigen from common subgroups (Karp, Kato, and Gilliam). However, variations in sensitivity and specificity arise due to these common subgroups. Immunochromatographic tests that detect IgM antibodies have sensitivities ranging from 74% to 90% and specificities from 86% to 99%.[21]
Polymerase chain reaction techniques: Polymerase chain reaction (PCR) techniques offer a significant advantage by identifying the disease before antibodies become detectable through serological methods, facilitating early diagnosis. While direct detection of the organism can be affected by antibiotics, positive results from eschar tissue cultures have been observed up to 7 days after antibiotic use.[1] Despite PCR's high sensitivity and specificity in detecting very low copy numbers, its cost remains a limiting factor for routine diagnosis in endemic areas.
Bacterial cultures: Bacterial cultures for O tsutsugamushi are challenging due to their obligate intracellular nature, requiring biosafety level 3 containment and significant technical expertise. Samples are typically obtained from the buffy coat of whole blood samples and skin biopsies from experimental animals. However, the long and intricate process makes culture impractical for routine clinical diagnosis. Therefore, it is primarily reserved for research purposes in specialized reference laboratories.
Cerebrospinal fluid evaluation: Cerebrospinal fluid (CSF) evaluation is beneficial for patients showing signs of central nervous system involvement in scrub typhus. Scrub meningitis can present with lymphocytic pleocytosis in CSF. The CSF findings in scrub typhus can resemble those seen in tuberculous meningitis (lymphocytic pleocytosis with elevated proteins) and viral meningoencephalitis.
Chest radiographs: Chest radiographs are recommended for patients presenting with respiratory symptoms in suspected scrub typhus cases. These images may reveal pleural effusions and pneumonia, including para-pneumonic effusions. Although relatively uncommon, ARDS characterized by bilateral heterogeneous diffuse infiltrates can be a severe complication of scrub typhus. Therefore, the diagnosis of scrub typhus relies heavily on maintaining a high level of clinical suspicion and using diagnostic tests judiciously, especially in areas with high endemicity.
Treatment / Management
Tetracycline, azithromycin, doxycycline, and rifampicin are all effective antimicrobials for treating scrub typhus. Studies have explored various dosing regimens for doxycycline and azithromycin, including a loading dose followed by maintenance doses. The average treatment duration for doxycycline is 7 days and for azithromycin is 3 days.[2] Additionally, tetracycline and rifampicin are also established as effective treatments for scrub typhus.(A1)
Antimicrobial Treatment for Scrub Typhus
Doxycycline: Doxycycline, commonly used as the primary treatment for most rickettsial diseases, is administered at 100 mg intravenously (IV) or orally twice daily for 7 to 14 days. Despite its efficacy, some reports have raised concerns about suspected doxycycline resistance.
Azithromycin: Azithromycin is an excellent alternative treatment option, especially in cases where resistance to doxycycline is suspected.[22] Due to its long tissue half-life and sustained post-antibiotic effects, azithromycin can be administered for a shorter duration while minimizing the risk of relapses. Additionally, its efficient penetration of polymorphonuclear leukocytes and macrophages, key target cells for O tsutsugamushi, enhances its efficacy.[23](A1)
Rifampicin: Rifampicin represents an alternative antimicrobial option for scrub typhus. However, clinicians must carefully consider the risk of inducing resistant tuberculosis in undiagnosed patients. Therefore, rifampicin should not be considered a first-line treatment choice but rather a second-line option after ruling out active tuberculosis.[24](A1)
Chemoprophylaxis
The use of prophylactic treatment with a weekly dose of 200 mg of doxycycline remains controversial, primarily recommended for individuals with a high occupational risk, such as agricultural laborers.[25] Despite these measures, some patients may still develop life-threatening complications such as MODS, necessitating an interprofessional approach to management involving ICU care. A recent multicentric randomized control trial has highlighted the effectiveness of IV combination therapy with doxycycline and azithromycin over 7 days for severe scrub typhus cases.[26] Patients in the ICU often require aggressive supportive measures such as IV fluids, while those with ARDS may need mechanical ventilation. However, timely administration of symptomatic treatment and appropriate antibiotic therapy can significantly contribute to patient recovery without enduring end-organ deficits.
Differential Diagnosis
Scrub typhus is one of the most underdiagnosed causes of tropical fevers, often presenting as a fever of unknown origin and causing diagnostic challenges similar to other rickettsioses. When scrub typhus manifests as encephalitis, it can be challenging to differentiate from common viral or bacterial causes of encephalitis. Due to overlapping symptoms, the presentation may be mistaken for flu-like syndromes related to other illnesses. Also, eschars, which occur in 10% to 90% of acute scrub typhus cases, can be easily overlooked.
Scrub typhus remains challenging to diagnose due to its potential to affect nearly every organ system. This often leads to delayed initiation of appropriate therapy despite a high index of clinical suspicion and comprehensive clinical knowledge. Differential diagnoses to consider include malaria, dengue, leptospirosis, and typhoid fever.
Prognosis
Studies focusing on severe scrub typhus have identified several deranged laboratory parameters correlating with a worse prognosis. These include leukocytosis, thrombocytopenia, elevated transaminases, abnormal chest x-rays, and elevated serum creatinine levels. Patients presenting with these features, along with septic shock and concomitant hypothermia, face a significantly higher risk of developing MODS and, ultimately, succumbing to the infection compared to those presenting with fever alone. Severe pulmonary involvement, such as in ARDS, is also strongly associated with increased mortality rates. Renal failure in severe scrub typhus cases can be life-threatening, with a serum creatinine level exceeding 1.4 mg serving as an independent predictor of fatality in scrub typhus.
Lee et al, in a retrospective epidemiological study, suggested that patients treated in the intensive care unit (ICU) presenting without an eschar and with higher APACHE II scores have independent risk factors associated with high mortality.[27] Furthermore, Sonthayanon et al demonstrated in their study that initial high loads of scrub typhus DNA at admission correlate positively with increased mortality and prolonged illness duration.[28] However, due to cost constraints, the practical value of DNA testing is limited in resource-limited settings. Notably, prognostic indicators for severe scrub typhus infection are yet to be conclusively established due to limited studies and conflicting results.
Severe scrub typhus manifestations such as MODS in the form of ARDS, myocarditis, liver failure, acute renal failure, encephalitis in various combinations, and shock from vasculitis are common presentations. Any delay in treatment can significantly increase the mortality index, which can sometimes be as high as 30%.
Complications
The complications associated with scrub typhus span across multiple organ systems, often reflecting the severity and systemic impact of the disease on affected individuals, as mentioned below.
Pulmonary
Pulmonary complications associated with scrub typhus include conditions such as ARDS and pneumonitis, often indicating severe disease. Common radiological abnormalities observed include bilateral reticular opacities and pleural effusion, along with concurrent cardiac issues such as congestive heart failure.[29][30] Scrub typhus patients may develop pleural effusions, which may be transudative or exudative and are more prevalent in older patients, those with cardiac complications, or individuals with reduced serum albumin levels.[13]
Cardiac
Cardiac involvement in scrub typhus lacks a clearly defined pathogenesis, and its implications remain uncertain. However, reversible cardiomegaly has been documented in over 80% of patients in certain autopsy studies.[31] Electrocardiogram (ECG) changes in scrub typhus patients span from sinus tachycardia to relative bradycardia (more common in scrub typhus). Atrial flutter or fibrillation, atrial standstill, heart block, PR-interval prolongation, ST-T changes, prominent U-waves, and sometimes ventricular premature beats and Q-T prolongation are seen.[32]
Inconclusive evidence suggests a higher severity or worse outcomes in most patients despite the varied ECG manifestations. ECG changes may result from electrolyte imbalances or acidosis rather than being a direct effect of scrub typhus infection. Myocarditis, although rare, can present severely with nonspecific symptoms such as fever, myalgia, palpitations, and exertional dyspnea to cardiogenic shock or sudden cardiac death.[14]
Neurological
The pathophysiology of nervous system involvement in scrub typhus infections may be multifactorial. However, the primary mechanism appears to be due to vasculitis and perivasculitis in the endothelial cells, where the proliferation of O tsutsugamushi occurs. Scrub typhus meningitis, as in other forms, can present with headache or nuchal rigidity and an altered sensorium or focal neurological deficits. Examination of the CSF may show pleocytosis; sometimes, results can mimic tuberculous meningitis, which can show lymphocytic pleocytosis with increased proteins.[11]
Meningoencephalitis, which is the more severe form of CNS involvement, is characterized by presentation with altered sensorium and seizures,[33][34] often seen in the context of MODS, leading to potential case fatalities due to systemic complications. Rarely, patients may exhibit unilateral or bilateral sixth nerve palsy. However, with appropriate treatment, CNS involvement typically has a favorable outcome. Additionally, in another spectrum, scrub typhus may also present with transient parkinsonism, myoclonus, opsoclonus, trigeminal neuralgia, and visual hallucinations.
Gastrointestinal
Gastrointestinal symptoms usually develop 3 to 7 days after the initiation of fever. Scrub typhus can present as an acute abdomen without an underlying surgical cause, and features like diarrhea, pancreatitis, gastrointestinal bleeding, and liver dysfunction manifested in the form of raised transaminases and bilirubin are not uncommon. Abdominal pain, abdominal tenderness, indigestion, nausea, and vomiting, along with splenomegaly, also happen frequently with scrub typhus.[17] The mechanism of these varied GI manifestations is unknown. However, as in other organ systems, the causative organism has been implicated in vasculitis and perivasculitis of the small blood vessels.[15] Scrub typhus patients without eschar often present with hepatic dysfunction, indicated by elevated liver enzymes.[16]
Renal
Acute kidney injury is an under-recognized complication of scrub typhus, and numerous factors contribute to its development. Acute kidney injury is a predictor of mortality, and possible mechanisms include pre-renal failure due to septic shock, vasculitis of the renal vessels, rhabdomyolysis, and direct renal invasion.[35] However, the prevalence of comorbidities was the significant predictor in patients who developed acute kidney injury, and prompt treatment with anti-rickettsial drug therapy and proper supportive care is very much essential to avert adverse outcomes.
Additional complications
Scrub typhus can rarely lead to severe complications such as disseminated intravascular coagulopathy and hemophagocytic syndrome. Patients may also experience papilledema, a common ocular manifestation, which typically occurs between the second and third week of illness and may persist into the convalescent period. Pregnant women with scrub typhus are at risk of adverse pregnancy outcomes, including preterm deliveries, miscarriages, and delivery of small-for-gestational-age babies. In severe cases, neonatal deaths have also been reported as a consequence of maternal scrub typhus infection.
Consultations
A primary clinician can diagnose and treat the majority of cases. However, complicated scrub typhus cases need input from an infectious disease clinician, especially for handling drug-resistant cases.
Deterrence and Patient Education
The symptoms of scrub typhus infection mimic those of various other diseases. Given its endemic nature in specific regions, patients should inform their clinicians of any recent travel to these areas if symptoms arise. Individuals should seek medical evaluation in endemic areas if they develop a fever with eschar formation. Emphasizing timely management is crucial, as scrub typhus is curable without complications. Travelers to endemic regions should be educated on preventing mite bites. Although chemoprophylaxis with 200 mg of doxycycline weekly can be considered for high-risk groups, its use remains controversial. Currently, a vaccine for preventing scrub typhus infection is unavailable.
Enhancing Healthcare Team Outcomes
The complex diagnosis and management of scrub typhus are best handled by an interprofessional team consisting of infectious disease experts, emergency department clinicians, primary clinicians, nurses, pharmacists, and skilled laboratory technicians. This collaboration provides participating clinicians with current recommendations for managing scrub typhus, underscoring the necessity of collaboration among various healthcare professionals involved in the diagnosis and management process.
Skilled laboratory technicians play a crucial role in the team when lab testing is available. Patients with severe scrub typhus require transfer to the ICU, where supportive care includes IV fluids, acetaminophen for fever management, and antibiotics such as doxycycline or azithromycin. A confirmed diagnosis is typically established through antigen detection, PCR techniques, or serological testing. However, no laboratory tests can predict the progression to severe disease. The prognosis for untreated scrub typhus is abysmal, but with appropriate care, most patients can survive, though they may experience residual multisystem organ damage in some cases.
Media
(Click Image to Enlarge)
References
Janardhanan J, Trowbridge P, Varghese GM. Diagnosis of scrub typhus. Expert review of anti-infective therapy. 2014 Dec:12(12):1533-40. doi: 10.1586/14787210.2014.974559. Epub 2014 Oct 31 [PubMed PMID: 25359599]
Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: A systematic review. PLoS neglected tropical diseases. 2017 Sep:11(9):e0005838. doi: 10.1371/journal.pntd.0005838. Epub 2017 Sep 25 [PubMed PMID: 28945755]
Level 1 (high-level) evidenceDevasagayam E, Dayanand D, Kundu D, Kamath MS, Kirubakaran R, Varghese GM. The burden of scrub typhus in India: A systematic review. PLoS neglected tropical diseases. 2021 Jul:15(7):e0009619. doi: 10.1371/journal.pntd.0009619. Epub 2021 Jul 27 [PubMed PMID: 34314437]
Level 1 (high-level) evidenceVarghese GM, Janardhanan J, Mahajan SK, Tariang D, Trowbridge P, Prakash JA, David T, Sathendra S, Abraham OC. Molecular epidemiology and genetic diversity of Orientia tsutsugamushi from patients with scrub typhus in 3 regions of India. Emerging infectious diseases. 2015 Jan:21(1):64-9. doi: 10.3201/eid2101.140580. Epub [PubMed PMID: 25530231]
Wongprompitak P, Anukool W, Wongsawat E, Silpasakorn S, Duong V, Buchy P, Morand S, Frutos R, Ekpo P, Suputtamongkol Y. Broad-coverage molecular epidemiology of Orientia tsutsugamushi in Thailand. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2013 Apr:15():53-8. doi: 10.1016/j.meegid.2011.06.008. Epub 2011 Jun 25 [PubMed PMID: 21712103]
Hanson B. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infection and immunity. 1985 Dec:50(3):603-9 [PubMed PMID: 2415453]
Level 3 (low-level) evidenceRanjan J, Prakash JAJ. Scrub typhus re-emergence in India: Contributing factors and way forward. Medical hypotheses. 2018 Jun:115():61-64. doi: 10.1016/j.mehy.2018.03.019. Epub 2018 Mar 31 [PubMed PMID: 29685200]
Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS neglected tropical diseases. 2017 Nov:11(11):e0006062. doi: 10.1371/journal.pntd.0006062. Epub 2017 Nov 3 [PubMed PMID: 29099844]
Rajapakse S, Rodrigo C, Fernando D. Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pacific journal of tropical medicine. 2012 Apr:5(4):261-4. doi: 10.1016/S1995-7645(12)60036-4. Epub [PubMed PMID: 22449515]
Level 3 (low-level) evidenceMunegowda KC, Nanda S, Varma M, Bairy I, Vidyasagar S. A prospective study on distribution of eschar in patients suspected of scrub typhus. Tropical doctor. 2014 Jul:44(3):160-2. doi: 10.1177/0049475514530688. Epub 2014 Apr 15 [PubMed PMID: 24737886]
Rana A, Mahajan SK, Sharma A, Sharma S, Verma BS, Sharma A. Neurological manifestations of scrub typhus in adults. Tropical doctor. 2017 Jan:47(1):22-25 [PubMed PMID: 27059055]
Kim DE, Lee SH, Park KI, Chang KH, Roh JK. Scrub typhus encephalomyelitis with prominent focal neurologic signs. Archives of neurology. 2000 Dec:57(12):1770-2 [PubMed PMID: 11115244]
Level 3 (low-level) evidenceRajapakse S, Weeratunga P, Sivayoganathan S, Fernando SD. Clinical manifestations of scrub typhus. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2017 Feb 1:111(2):43-54. doi: 10.1093/trstmh/trx017. Epub [PubMed PMID: 28449088]
Ki YJ, Kim DM, Yoon NR, Kim SS, Kim CM. A case report of scrub typhus complicated with myocarditis and rhabdomyolysis. BMC infectious diseases. 2018 Nov 7:18(1):551. doi: 10.1186/s12879-018-3458-1. Epub 2018 Nov 7 [PubMed PMID: 30404620]
Level 3 (low-level) evidenceSv PD, M A, Kumar AC, Krishna Reddy H, Bl S, Siva Kumar V. Acute pancreatitis associated with scrub typhus. Tropical doctor. 2017 Jan:47(1):65-67 [PubMed PMID: 27411365]
Su TH, Liu CJ, Shu PY, Fu YH, Chang CH, Jao P, Kao JH. Associated factors and clinical implications of serum aminotransferase elevation in scrub typhus. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2016 Dec:49(6):941-946. doi: 10.1016/j.jmii.2014.10.005. Epub 2014 Nov 11 [PubMed PMID: 25556044]
Level 2 (mid-level) evidenceKim SJ, Chung IK, Chung IS, Song DH, Park SH, Kim HS, Lee MH. The clinical significance of upper gastrointestinal endoscopy in gastrointestinal vasculitis related to scrub typhus. Endoscopy. 2000 Dec:32(12):950-5 [PubMed PMID: 11147943]
Shivalli S. Diagnostic evaluation of rapid tests for scrub typhus in the Indian population is needed. Infectious diseases of poverty. 2016 May 12:5(1):40. doi: 10.1186/s40249-016-0137-6. Epub 2016 May 12 [PubMed PMID: 27169486]
Jang WJ, Huh MS, Park KH, Choi MS, Kim IS. Evaluation of an immunoglobulin M capture enzyme-linked immunosorbent assay for diagnosis of Orientia tsutsugamushi infection. Clinical and diagnostic laboratory immunology. 2003 May:10(3):394-8 [PubMed PMID: 12738637]
Level 2 (mid-level) evidenceBlacksell SD, Lim C, Tanganuchitcharnchai A, Jintaworn S, Kantipong P, Richards AL, Paris DH, Limmathurotsakul D, Day NPJ. Optimal Cutoff and Accuracy of an IgM Enzyme-Linked Immunosorbent Assay for Diagnosis of Acute Scrub Typhus in Northern Thailand: an Alternative Reference Method to the IgM Immunofluorescence Assay. Journal of clinical microbiology. 2016 Jun:54(6):1472-1478. doi: 10.1128/JCM.02744-15. Epub 2016 Mar 23 [PubMed PMID: 27008880]
Anitharaj V, Stephen S, Pradeep J, Park S, Kim SH, Kim YJ, Kim EY, Kim YW. Serological Diagnosis of Acute Scrub Typhus in Southern India: Evaluation of InBios Scrub Typhus Detect IgM Rapid Test and Comparison with other Serological Tests. Journal of clinical and diagnostic research : JCDR. 2016 Nov:10(11):DC07-DC10. doi: 10.7860/JCDR/2016/24051.8861. Epub 2016 Nov 1 [PubMed PMID: 28050364]
Phimda K, Hoontrakul S, Suttinont C, Chareonwat S, Losuwanaluk K, Chueasuwanchai S, Chierakul W, Suwancharoen D, Silpasakorn S, Saisongkorh W, Peacock SJ, Day NP, Suputtamongkol Y. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrobial agents and chemotherapy. 2007 Sep:51(9):3259-63 [PubMed PMID: 17638700]
Level 1 (high-level) evidenceDinos GP. The macrolide antibiotic renaissance. British journal of pharmacology. 2017 Sep:174(18):2967-2983. doi: 10.1111/bph.13936. Epub 2017 Aug 10 [PubMed PMID: 28664582]
Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet (London, England). 2000 Sep 23:356(9235):1057-61 [PubMed PMID: 11009140]
Level 1 (high-level) evidenceHarris PNA, Oltvolgyi C, Islam A, Hussain-Yusuf H, Loewenthal MR, Vincent G, Stenos J, Graves S. An outbreak of scrub typhus in military personnel despite protocols for antibiotic prophylaxis: doxycycline resistance excluded by a quantitative PCR-based susceptibility assay. Microbes and infection. 2016 Jun:18(6):406-411. doi: 10.1016/j.micinf.2016.03.006. Epub 2016 Mar 19 [PubMed PMID: 27005452]
Varghese GM, Dayanand D, Gunasekaran K, Kundu D, Wyawahare M, Sharma N, Chaudhry D, Mahajan SK, Saravu K, Aruldhas BW, Mathew BS, Nair RG, Newbigging N, Mathew A, Abhilash KPP, Biswal M, Prasad AH, Zachariah A, Iyadurai R, Hansdak SG, Sathyendra S, Sudarsanam TD, Prakash JAJ, Manesh A, Mohan A, Tarning J, Blacksell SD, Peerawaranun P, Waithira N, Mukaka M, Cheah PY, Peter JV, Abraham OC, Day NPJ, INTREST Trial Investigators. Intravenous Doxycycline, Azithromycin, or Both for Severe Scrub Typhus. The New England journal of medicine. 2023 Mar 2:388(9):792-803. doi: 10.1056/NEJMoa2208449. Epub [PubMed PMID: 36856615]
Lee CS, Hwang JH, Lee HB, Kwon KS. Risk factors leading to fatal outcome in scrub typhus patients. The American journal of tropical medicine and hygiene. 2009 Sep:81(3):484-8 [PubMed PMID: 19706919]
Level 2 (mid-level) evidenceSonthayanon P, Chierakul W, Wuthiekanun V, Phimda K, Pukrittayakamee S, Day NP, Peacock SJ. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. Journal of clinical microbiology. 2009 Feb:47(2):430-4. doi: 10.1128/JCM.01927-08. Epub 2008 Dec 17 [PubMed PMID: 19091812]
Choi YH, Kim SJ, Lee JY, Pai HJ, Lee KY, Lee YS. Scrub typhus: radiological and clinical findings. Clinical radiology. 2000 Feb:55(2):140-4 [PubMed PMID: 10657161]
Level 2 (mid-level) evidenceSong SW, Kim KT, Ku YM, Park SH, Kim YS, Lee DG, Yoon SA, Kim YO. Clinical role of interstitial pneumonia in patients with scrub typhus: a possible marker of disease severity. Journal of Korean medical science. 2004 Oct:19(5):668-73 [PubMed PMID: 15483341]
Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, Lee SH. Scrub typhus: clinical, pathologic, and imaging findings. Radiographics : a review publication of the Radiological Society of North America, Inc. 2007 Jan-Feb:27(1):161-72 [PubMed PMID: 17235005]
Thipmontree W, Tantibhedhyangkul W, Silpasakorn S, Wongsawat E, Waywa D, Suputtamongkol Y. Scrub Typhus in Northeastern Thailand: Eschar Distribution, Abnormal Electrocardiographic Findings, and Predictors of Fatal Outcome. The American journal of tropical medicine and hygiene. 2016 Oct 5:95(4):769-773 [PubMed PMID: 27573633]
Pradhan B, Jindal A. Acute encephalitis syndrome following scrub typhus infection. Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2014 Oct:18(10):700-1. doi: 10.4103/0972-5229.142184. Epub [PubMed PMID: 25316985]
Saifudheen K, Kumar KG, Jose J, Veena V, Gafoor VA. First case of scrub typhus with meningoencephalitis from Kerala: An emerging infectious threat. Annals of Indian Academy of Neurology. 2012 Apr:15(2):141-4. doi: 10.4103/0972-2327.95002. Epub [PubMed PMID: 22566732]
Level 3 (low-level) evidenceSun IO, Kim MC, Park JW, Yang MA, Lee CB, Yoon HJ, Kim JG, Lee KY. Clinical characteristics of acute kidney injury in patients with scrub typhus--RIFLE criteria validation. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2014 Feb:20(2):93-6. doi: 10.1016/j.jiac.2013.08.007. Epub 2013 Dec 12 [PubMed PMID: 24485324]
Level 1 (high-level) evidence